1.IntroductionCorneal ablation by an argon fluoride (ArF) excimer laser [wavelength of 193 nm, pulse width of 12 ns (FWHM)], which enables highly reliable shaping of corneas, has been used in photorefractive keratectomy (PRK) and laser-assisted in situ keratomileusis (LASIK).1 To monitor the ablation process during corneal surgery, we developed a noncontact, noninvasive temperature measurement system based on thermal radiometry with a time response of 15.7 ns.2 We experimentally demonstrated for the first time that the rise in temperature of the corneal surface during ArF excimer laser ablation depends on the fluence. Temperatures reaching over 100°C at the fluence are used clinically. This high temperature suggests that superheating occurs during ArF excimer laser ablation of the cornea. We have conducted preliminary experiments to study the influence of laser ablation on corneal tissue, using sections stained with hematoxylin and eosin that we analyzed through an optical microscope.3 After ArF excimer laser ablation, no irregularities or coagulation could be seen on the exposed stromal surface. Thus, ablation seems to have had no apparent thermal effect on the cornea in our study. However, since the optical penetration depth for corneal tissue at 193 nm is around 1 μm, this outcome was thought to have resulted from the limited resolution of the optical microscope, because thermal effects had actually occurred. Our previous measurements indicated that the temperature of the corneal surface increased rapidly during laser irradiation and then decreased relatively slowly.2 4 We therefore decided that the corneal reaction after ArF excimer laser ablation, which can influence the internal properties of the cornea, should be evaluated. Corneal cell-level reaction has been used as an important indicator to demonstrate the validity of treatments using various instruments and techniques for PRK and LASIK.5 We selected heat shock protein (HSP 70) and apoptosis to immunohistochemically study corneal cell-level reaction after laser ablation. HSP 70 appears in response to environmental stress to protect damaged cells.6 HSPs are classified into five groups according to their molecular weights. HSP 70, whose molecular weight is about 70,000, plays an essential role in protecting tissue from thermal stress.7 8 HSP 72, inducible HSP 70, has been reported to be synthesized in response to a wide variety of environmental temperatures.9 10 On the other hand, apoptosis, programmed cell death, plays a critical role in the regulation of tissue homeostasis, such as in the maintenance of tissue structure and cellular reorganization.11 Thus, apoptosis has been regarded as an indicator of homeostasis. In the present study, we immunohistochemically observed the appearances of HSP 72 and apoptosis at various laser ablation fluences as a function of time after laser ablation. We conducted high-speed temperature measurements and spectral observation of optical emissions during ArF excimer laser ablation of the cornea. There have been no experimental studies on the relationship between corneal cell-level reaction after laser ablation and the physical parameters directly related to the ablation mechanism, such as temperature rise and optical emission spectra. Special emphasis was placed on the effect of fluence-dependent physical parameters on expressions of HSP 72 and apoptosis in the cornea. 2.Materials and Methods2.1.Animal Ablation ExperimentsAnimal experiments were conducted according to the protocols approved by the animal committee of the National Defense Medical College. We immunohistochemically studied 34 eyes of 17, 12-week-old, white Japanese rabbits with body weights ranging from 2.5 to 3 kg. They were anesthetized with intramuscular injections of ketamine (50 mg/kg) and xylazine (20 mg/kg) before being ablated with an ArF excimer laser. We used Thom’s experimental method12 for the treatment. A pulsed ArF excimer laser (L4500 produced by Hamamatsu Photonics Co., Ltd., Shizuoka, Japan) at a wavelength of 193 nm with a pulse width of 12 ns (FWHM) was used for the corneal ablation. The laser beam was passed through a rectangular aperture (22×6 mm) to eliminate the amplified spontaneous emission (ASE) component and focused on the sample through a spherical silica lens with a focal length of 200 mm. The area of the corneal surface subjected to laser irradiation was 7×2 mm for all eyes. The laser pulse energy was adjusted with a variable attenuator, and measured with a power meter (10A-P produced by OPHIR Optronics Ltd. Maine). The repetition rate of the laser was 10 Hz and there were a total of 499 laser pulses for each ablation.12 The epithelium was removed by ablation in the present study. The eyes were divided into four categories: those subjected to ablation with a fluence of 250 mJ/cm2 which is higher than the fluence range used clinically (group A, 10 eyes); those subjected to ablation with a fluence of 180 mJ/cm2, the fluence range used clinically (group B, 10 eyes); those subjected to ablation with a fluence of 100 mJ/cm2, which is lower than the fluence used clinically (group C, 10 eyes); and a control group without ablation (4 eyes). These fluences (250, 180, and 100 mJ/cm2) were chosen based on our previous study of measuring ablation depth at various fluences.4 The ablation depths (etch depths) per pulse at fluences of 250, 180, and 100 mJ/cm2 were 0.69, 0.55, and 0.19 μm, respectively. 2.1.1.Immunohistochemical studyAfter laser ablation, the rabbit eyes were irrigated with balanced saline and steroids and mitomycin were topically applied to reduce stromal haze.13 Mytomycin ointment was applied to all eyes twice a day for 2 days after ablation. The same procedures were used in the ablation groups and the control group. Seven of the rabbits were sacrificed 2 days after ablation with an overdose of pentobarbital sodium, and the remaining ten rabbits were sacrificed 7 days after the procedure of Hong and Lee.14 The corneas were immediately excised and fixed in Carnoy’s solution for 4 h. They were then dehydrated in an ethanol solution, paraffin embedded, and sectioned at thicknesses of 4 μm. To immunohistochemically study the inducible heat shock protein 70 (HSP 72), a monoclonal antibody directed against the inducible form of the protein was used (mouse IgG 1, SPA-810, Stress Gen, Victoria, Canada). This mouse monoclonal antibody has a cross-reaction with rabbits. The sections were incubated overnight with a 1:200 dilution of the monoclonal antibody at 4°C. Antigen–antibody complexes were visualized using an avidin-biotin Vectastain ABC Elite Kit (Vector Laboratories, California). Color development was done with a 3,3′-diaminobenzidine (DAB) solution using a DAB substrate kit (SK-4100, Vector Laboratories). Mayers’ hematoxylin was used as the counterstain. In this study, only inducible HSP 70 (HSP 72) was detected, while constitutive HSP 70 (HSP73) was not, owing to the specificity of the monoclonal antibody.15 16 All specimens were observed through an optical microscope with an objective lens (×40) (Nikon, Japan). We counted the numbers of positive-stained cells of HSP 72 within four randomly selected microscopic fields in the epithelium of samples in groups A to C. We also counted the total number of cells in the epithelium of each sample. We then calculated the ratio of the number of positive-stained cells to the total number of cells in the observed field. The apoptosis was evaluated by terminal deoxyribonucleotidyl transferase (TDT)-mediated deoxyuridine triphosphate (dUTP)-digoxigenin nick end labeling (TUNEL) analysis using ApopTag (S7101, Intergen Co. New York). The TUNEL assay enabled the DNA fragmentation associated with apoptosis to be detected. 2.1.2.Temperature and optical emission measurementsExcised pieces of the corneal buttons of 22 rabbit eyes were placed on saline-moistened gauze to keep the samples hydrated. The schematics for the experimental arrangement are shown in Fig. 1. We recently reported on the usefulness of a high-speed temperature measurement system using a technique that allows thermal radiation to be detected with a response time of 15.7 ns (Ref. 2). Thermal radiation from the corneal surface during ablation was detected with a photovoltaic mercury-cadmium-tellurium (HgCdTe) detector that had an antireflection germanium-coated window. To determine the temperature of the corneal surface from the measured thermal radiation signal, the measurement system was calibrated using a silicon rubber heater for the temperature standard. We then determined the time courses of corneal surface temperature at various laser ablation fluences. Thermal doses were derived from the measured time courses of temperature using Arrhenius’s theory (Eq. 1).17 where A is the preexponential factor, E is the activation energy, R is the universal gas constant, and T is the corneal surface temperature. The thermal doses were normalized using those in group A.Figure 1A system to measure temperature and optical emission spectra. L1, silica lens; L2, zinc-selenium lens; M1; gold-coated concave mirror; PM; power meter. The output signal from a biplanar phototube is used to trigger temperature measurements. 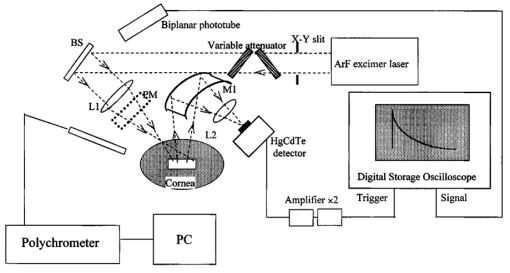 The laser-induced optical emission was measured, together with the temperature. The time-integrated optical emission spectrum was measured with a fiber-coupled polychromator (PMA-11 C5966011 produced by Hamamatsu Photonics Co., Ltd., Shizuoka, Japan`). The exposure time of the polychromator was 10 ms. 3.ResultsThe appearance of HSP 72 depended on the fluence and time after ablation, as Figs. 2(a) to 2(d) and Figs. 3(a) to 3(c) show. In the control group without laser irradiation [Fig. 2(d)], HSP 72 was not detected. Figures 2(a) to 2(c) reveal that HSP 72 is only localized in the regenerative epithelium in all ablation groups 2 days after ablation. HSP 72 could not be detected in the stroma. In Fig. 2(a), the sample in group A (fluence of 250 mJ/cm2) that was examined 2 days after ablation shows a very dense expression of HSP 72 over the entire regenerative epithelium. In Fig. 2(b), the sample in group B (fluence of 180 mJ/cm2) exhibits localization of HSP 72 mainly at the boundary between the stroma and the regenerative epithelium, while less HSP 72 appears in the upper layer of the regenerative epithelium. In Fig. 2(c) the sample in group C (fluence of 100 mJ/cm2) has less HSP 72 at the boundary between the stroma and the regenerative epithelium compared with the amounts of HSP 72 in samples in groups A and B. Figure 2A comparison of localization of HSP 72 in ablation groups and control group 2 days after ablation. The arrows indicate HSP 72 expressions. HSP 72 is stained brown. The violet stain is a counterstain produced by hematoxylin. RE, ST, and EP indicate regenerative epithelium, stroma, and epithelium, respectively. Scale bar=75 μm. (a) Ablation with fluence of 250 mJ/cm2. Very dense staining can be seen over the entire regenerative epithelium. (b) Ablation with fluence of 180 mJ/cm2. Stained cells and extracellular matrix are localized at the boundary between the stroma and regenerative epithelium. (c) Ablation with fluence of 100 mJ/cm2. A few stained cells appear at the boundary. (d) Control without ablation. HSP 72 is invisible. 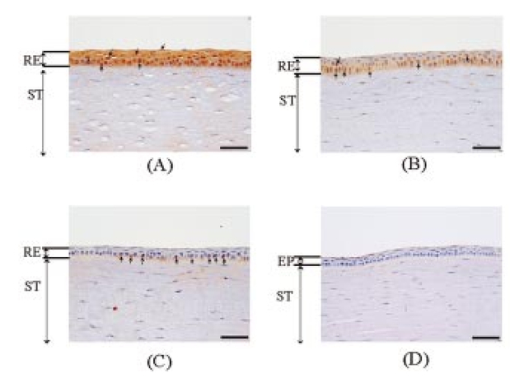 Figure 3A comparison of localizations of HSP 72 in ablation groups and control group 7 days after ablation. The arrows indicate HSP 72 expressions. HSP 72 is stained brown. The violet stain is a counterstain produced by hematoxylin. RE and ST indicate regenerative epithelium and stroma. Scale bar=75 μm. (a) Ablation with fluence of 250 mJ/cm2. HSP 72 is localized in the stroma and at the boundary between the stroma and regenerative epithelium. (b) Ablation with fluence of 180 mJ/cm2. The intensity of staining is less than in the group A sample. (c) Ablation with fluence of 100 mJ/cm2. HSP 72 is not visible. 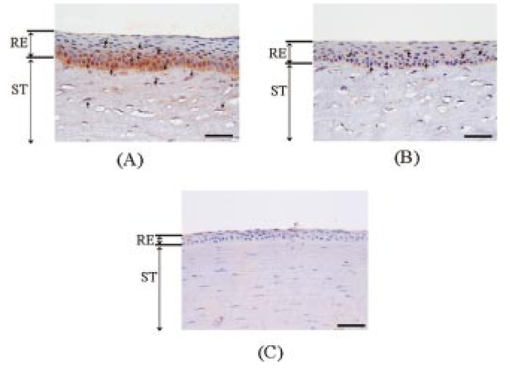 The results obtained 7 days after ablation are shown in Fig. 3. The expression of HSP 72 is localized at the boundary between the stroma and regenerative epithelium [Fig. 3(a)], and is also observed up to a depth of a few hundred micrometers in the stroma. We could only see HSP 72 in the deep stroma in group A. HSP 72 is less expressed in the upper layer of the regenerative epithelium. Figure 3(b) reveals a substantial amount of HSP 72 at the boundary between the stroma and regenerative epithelium and a smaller amount of HSP 72 in the upper layer of the regenerative epithelium. There is little expression of HSP 72 in Fig. 3(c). Table 1 lists the regenerative epithelium thicknesses. The epithelium increases in thickness with increasing fluence and time elapsed after ablation. Table 1
The dependence of the HSP 72 positive cell ratios in regenerative epithelium on thermal dose and the time after ablation are summarized in Fig. 4. The ratios increase with increased thermal dose in all samples examined 2 and 7 days after ablation. The ratios increase dramatically when the thermal dose is increased from 0.29 to 0.71. The ratios of positive-stained cells are higher in samples examined 2 days after ablation than in samples examined 7 days after the procedure. A better comparison of the expression of HSPs in the three groups might have been possible if the areas of stained HSP 72 had been measured instead of counting the number of positive-stained cells. Figure 4The positive cell ratio of HSP 72 as function of thermal dose. The bars represent standard deviations. The positive cell ratio increases with increasing fluence; it increases dramatically from 100 to 180 mJ/cm2. 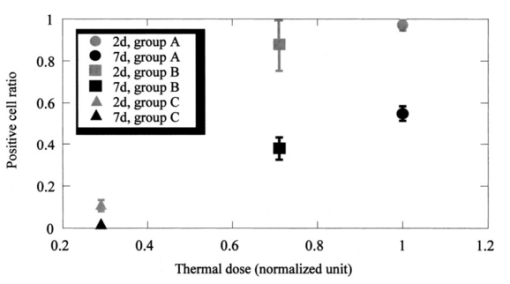 Figure 5 shows the expression of TUNEL-positive cells, which appear in the stroma and regenerative epithelium in group A (fluence of 250 mJ/cm2) 2 days after ablation. In the control group, TUNEL-positive cells could only be found in the epithelium, mainly in the superficial epithelium. Apoptosis could not be found in the deep stroma in groups B or C (data not shown), or in the control group; it was only found in group A. Figure 5A comparison of localizations of TUNEL-positive cells in the control group and the group subjected to ablation with a fluence of 250 mJ/cm2, conducted 2 days after ablation. The arrows indicate TUNEL-positive cells, which are stained black. RE, ST, and EP indicate regenerative epithelium, stroma, and epithelium, respectively. Scale bar=75 μm. (a) Ablation with fluence of 250 mJ/cm2. Apoptosis is localized in the stroma and regenerative epithelium. (b) Control without ablation. Apoptosis is localized in the epithelium. 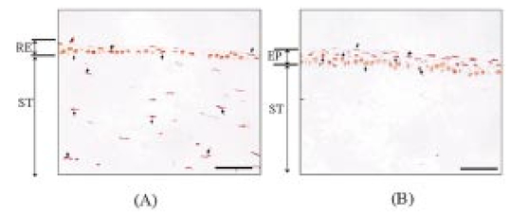 Figure 6 shows the time-integrated laser-induced optical emission spectra during ablation. Optical emission in this study means laser-induced fluorescence and optical breakdown.18 There are large differences in both the emission intensities and spectra [Figs. 6(a) to 6(c)]. The spectra [Fig. 6(a)] reveal a pronounced peak at around 580 nm, which is considered to have been caused by optical breakdown. Fluorescence emissions are the most intense in Fig. 6(a) and the second most intense in Fig. 6(b). The spectrum for fluorescence is similar to that observed in previous studies19 and shows a broad peak centered at around 300 nm. Figure 6A comparison of the spectra of time-integrated laser-induced optical emissions during corneal ablation by an ArF excimer laser for different fluences. The exposure time of the polychromator was 10 ms. (a) Ablation with fluence of 250 mJ/cm2. (b) Ablation with fluence of 180 mJ/cm2. (c) Ablation with fluence of 100 mJ/cm2. 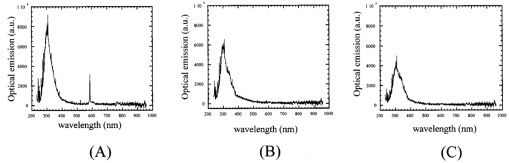 4.DiscussionHSP 72 only appeared in samples exposed to the laser, indicating that this was caused by irradiation. Also, it mainly appeared in the regenerative epithelium, not in the stroma. In fact, its appearance in the deep stroma was only observed in the group A samples 7 days after ablation. This finding is consistent with the previous observations reported by Kitazawa et al.;13 and Hong et al.;,14 but the mechanism behind HSP 72 being expressed in the regenerative epithelium is still unknown. In a dermatological study, Laplante et al.;20 reported on localization of HSP 72 in regenerative tissue. They found expressions of HSP 27, 60, 70, and 90 in the regenerative epidermis of a mouse during the healing process after a wound had been made through all layers of the skin. This means that the expression of HSP 70, as a repair function, correlates with cell proliferation in regenerative tissue,21 which supports our observation of HSP 72 in the regenerative epithelium. In our study, HSP 72 appeared as a repair function after the epithelium and a part of the stroma were ablated, and the amount of this heat shock protein in the regenerative epithelium correlated with the thermal dose. There was also a positive correlation between the thickness of the epithelium and the amount of HSP 72. These correlations between the amount of HSP 72, the thickness of the epithelium, and the thermal dose were verified by nonparametric tests and statistical analyses (no detailed data are given in this study). There appear to be no reports on experiments that correlate the amount of HSP 72 in the regenerative epithelium with the thermal dose estimated by high-speed temperature measurement. Our present study may help us to better understand the mechanism behind the expression of HSP 72. Beere and Green22 reported that the expression of HSP 72 inhibited the appearance of apoptosis. Because our study revealed that both HSP 72 and apoptosis localized up to a depth of a few hundred micrometers in the stroma in group A, we could not confirm that apoptosis was inhibited. The localization of apoptosis was also reported by Helena et al.;11 and their results exhibited a tendency similar to ours. Apoptosis normally occurs in the epithelium because the turnover is short.23 In our study, we only observed apoptosis and HSP 72 in the deep stroma at a fluence with an optical breakdown in the spectra measured during laser ablation. Therefore, our observations suggest that the occurrence of apoptosis and HSP 72 in the stroma is strongly related to optical breakdown. The appearance of HSP 72 in the cornea after ArF excimer laser ablation may be caused by a temperature rise in the cornea, irradiation by UV light, or shock waves induced by optical breakdown. That is, shock waves may induce apoptosis and HSP 72 in the stroma. In fact, HSP 72 was localized up to a depth of a few hundred micrometers in the stroma in group A. This localization cannot be explained by the temperature rise or irradiation by UV light. As the optical penetration depth of the cornea at 193 nm is less than 1 μm,24 realistically, the temperature rise and UV irradiation may not have an influence to a depth of a few hundred micrometers in the stroma, even if we consider thermal conduction after the temperature increases. Through experimentation, we were able to examine both the corneal cell-level reaction and physical parameters directly related to the ablation mechanism for the first time. 5.ConclusionWe immunohistochemically studied the localization of HSP 72 and apoptosis in the corneas of rabbits 2 and 7 days after they had been subjected to ArF excimer laser ablation at fluences ranging from 100 to 250 mJ/cm2. The intensity of HSP 72 staining 2 and 7 days after ablation increased with increasing thermal dose, which resulted from high-speed temperature measurement. The measurement of optical emission spectra indicated that the apoptosis and HSP 72 expressed in the stroma were strongly related to the shock waves induced by optical breakdown. Although we need more data to study the relation between laser ablation and cell-level reaction, we emphasize that there may be a fluence-dependent mechanism of appearance of HSP 72 and apoptosis in the cornea following ArF excimer laser ablation. REFERENCES
Q. Ren
,
R. H. Keates
,
R. A. Hill
, and
M. W. Berns
,
“Laser refractive surgery: a review and current status,”
Opt. Eng. , 34 642
–660
(1995). Google Scholar
M. Ishihara
,
T. Arai
,
S. Sato
,
Y. Morimoto
,
M. Obara
, and
M. Kikuchi
,
“Measurement of the surface temperature of the cornea during ArF excimer laser ablation by thermal radiometry with a 15-ns time response,”
Lasers Surg. Med. , 30 54
–59
(2002). Google Scholar
M. Ishihara
,
T. Arai
,
S. Sato
,
Y. Morimoto
,
M. Obara
, and
M. Kikuchi
,
“The UV nano-second pulsed laser ablation of cornea,”
Proc. 21st Annual Meeting of the Laser Society of Japan , 21 222
(2001). Google Scholar
M. Ishihara
,
T. Arai
,
S. Sato
,
Y. Morimoto
,
M. Obara
, and
M. Kikuchi
,
“Temperature measurement for energy-efficient ablation by thermal radiation with a microsecond time constant from the corneal surface during ArF excimer laser ablation,”
Front Med. Biol. Eng. , 11
(3), 167
–175
(2001). Google Scholar
K. D. Hanna
,
Y. Pouliquen
,
G. O. Waring
,
M. Savoldelli
,
J. Cotter
,
K. Morton
, and
M. Menasche
,
“Corneal stromal wound healing in rabbits after 193-nm excimer laser surface ablation,”
Arch. Ophthalmol. (Chicago) , 107
(6), 895
–901
(1989). Google Scholar
K. Yamaguchi
,
M. F. Barbe
,
I. R. Brown
, and
M. Tytell
,
“Induction of stress (heat shock) protein 70 and its mRNA in rat corneal epithelium by hyperthermia,”
Curr. Eye Res. , 9
(9), 913
–918
(1990). Google Scholar
G. C. Li
and
J. Y. Mak
,
“Re-induction of HSP 70 synthesis: an assay for thermotolerance,”
Int. J. Hyperthermia , 5
(3), 389
–403
(1989). Google Scholar
R. H. Burdon
,
“Heat shock and the heat shock proteins,”
Biochem. J. , 240
(2), 313
–324
(1986). Google Scholar
S. P. Tomasovic
,
P. A. Steck
, and
D. Heitzman
,
“Heat-stress proteins and thermal resistance in rat mammary tumor cells,”
Radiat. Res. , 95
(2), 399
–413
(1983). Google Scholar
D. J. Blom
,
I. De Waard-Siebinga
,
R. S. Apte
,
G. P. Luyten
,
J. Y. Niederkorn
, and
M. J. Jager
,
“Effect of hyperthermia on expression of histocompatibility antigens and heat-shock protein molecules on three human ocular melanoma cell lines,”
Melanoma Res. , 7
(2), 103
–109
(1997). Google Scholar
M. C. Helena
,
F. Baerveldt
,
W. J. Kim
, and
S. E. Wilson
,
“Keratocyte apoptosis after corneal surgery,”
Invest. Ophthalmol. Visual Sci. , 39
(2), 276
–283
(1998). Google Scholar
S. B. Thom
,
J. S. Myers
,
C. J. Rapuano
,
R. C. Eagle Jr.,
S. B. Siepser
, and
J. A. Gomes
,
“Effect of topical anti-transforming growth factor-beta on corneal stromal haze after photorefractive keratectomy in rabbits,”
J. Cataract Refractive Surg. , 23
(9), 1324
–1330
(1997). Google Scholar
Y. Kitazawa
,
T. Tokoro
,
S. Ito
, and
Y. Ishii
,
“The efficacy of cooling on excimer laser photorefractive keratectomy in the rabbit eye,”
Surv. Ophthalmol. , 42 S82
–S88
(1997). Google Scholar
J. W. Hong
and
T. S. Lee
,
“The relationship between rabbit corneal opacity and immunohistochemical expression of heat shock protein 72/73 and c-fos after excimer laser photorefractive keratectomy,”
Ophthalmic Res. , 31
(3), 203
–209
(1999). Google Scholar
A. Capon
,
E. Souil
,
B. Gauthier
,
C. Sumian
,
M. Bachelet
,
B. Buys
,
B. S. Polla
, and
S. Mordon
,
“Laser assisted skin closure (LASC) by using a 815-nm diode-laser system accelerates and improves wound healing,”
Lasers Surg. Med. , 28
(2), 168
–175
(2001). Google Scholar
E. Souil
,
A. Capon
,
S. Mordon
,
A. T. Dinh-Xuan
,
B. S. Polla
, and
M. Bachelet
,
“Treatment with 815-nm diode laser induces long-lasting expression of 72- kDa heat shock protein in normal rat skin,”
Br. J. Dermatol. , 144
(2), 260
–266
(2001). Google Scholar
W. C. Dewey
,
“Arrhenius relationships from the molecule and cell to the clinic,”
Int. J. Hyperthermia , 10
(4), 457
–483
(1994). Google Scholar
S. Sato
,
M. Ogura
,
M. Ishihara
,
S. Kawauchi
,
T. Arai
,
T. Matsui
,
A. Kurita
,
M. Obara
,
M. Kikuchi
, and
H. Ashida
,
“Nanosecond, high-intensity pulsed laser ablation of myocardium tissue at the ultraviolet, visible, and near-infrared wavelengths: in-vitro study,”
Lasers Surg. Med. , 29
(5), 464
–473
(2001). Google Scholar
H. Nakano
,
M. Ishihara
,
T. Arai
,
S. Sato
,
M. Kikuchi
, and
M. Obara
,
“Development of a temperature monitoring method by means of auto-fluorescence measurement during ArF excimer laser irradiation,”
J. Jap. Soc. Las. Surg. Med. , 21
(4), 309
–317
(2000). Google Scholar
A. F. Laplante
,
V. Moulin
,
F. A. Auger
,
J. Landry
,
H. Li
,
G. Morrow
,
R. M. Tanguay
, and
L. Germain
,
“Expression of heat shock proteins in mouse skin during wound healing,”
J. Histochem. Cytochem. , 46
(11), 1291
–1301
(1998). Google Scholar
M. J. Schlesinger
,
“Heat shock proteins: the search for functions,”
J. Cell Biol. , 103
(2), 321
–325
(1986). Google Scholar
H. M. Beere
and
D. R. Green
,
“Stress management–heat shock protein-70 and the regulation of apoptosis,”
Trends Cell Biol. , 11
(1), 6
–10
(2001). Google Scholar
H. Ren
and
G. Wilson
,
“Apoptosis in the corneal epithelium,”
Invest. Ophthalmol. Visual Sci. , 37
(6), 1017
–1025
(1996). Google Scholar
G. H. Pettit
and
M. N. Ediger
,
“Corneal-tissue absorption coefficients for 193- and 213-nm ultraviolet radiation,”
Appl. Opt. , 35
(19), 3386
–3391
(1996). Google Scholar
|
||||||||||||||||||||
CITATIONS
Cited by 8 scholarly publications.
Laser ablation
Cell death
Excimer lasers
Temperature metrology
Cornea
Proteins
Laser optics

