|
|
|
Noninvasive imaging offers critically important windows into cell and tissue structure and function, normal and diseased physiology, and responses to therapeutic intervention. An explosion of imaging methods has occurred in recent decades, and applications span a wide range of biomedical specialties. Optical imaging, while limited with respect to the depths it can probe, offers exquisite spatial resolution and molecular specificity. In vivo applications of optical coherence and fluorescence tomography, reflectance and fluorescence confocal imaging, and multiphoton imaging are all undergoing rapid expansion.1, 2 Biologically important optical signals arise from endogenous chromophores and light-matter interactions3 and from exogenously administered labels targeting molecular or cellular structure or function.4, 5 Trafficking of immune cells in normal and malignant tissue is an area of intense interest, in which conventional technologies such as immunohistochemistry have provided extensive information. New in vivo techniques based on intravital confocal and multiphoton microscopy have revealed amazing images of immune cell interactions, such as those between antigen-presenting dendritic cells (DCs) and T cells, in surgically exposed lymph nodes.6, 7, 8, 9 Exploiting the unique morphology of dendritic cells, a recent study reported the use of reflectance confocal microscopy to image the distribution of epithelial DCs in human cornea in vivo.10 Langerhans cells (LCs) and other DCs in the skin are important, as they are often the first to encounter antigen. These cells are of particular consideration from an imaging standpoint in that they are directly accessible via optical methods. Recently, we demonstrated a fresh whole-mount immunolabeling method that enables multicolor imaging of tumor and normal tissue vasculature and lymphatics, host cell populations, and extracellular matrix with minimal perturbation of tissue architecture.11 Here, we describe the extension of a fluorophore-conjugated-antibody-labeling-based confocal fluorescence imaging method to living mice and use the technique to identify LC and dermal or interstitial DC (iDC) trafficking in normal ear and in an intradermal murine tumor model. Within after direct intradermal injection of antibody, the method yields images with very high contrast and confocal image resolution to depths of at least in living tissue. Excellent staining persists for a minimum of following a single injection. Although these two DC populations are labeled using the same antibody directed against the major histocompatibility complex class II (MHC-II), they are distinguished unambiguously on the basis of their distinct morphologies and their stratification in the epidermal and dermal layers of the skin.12 Prior to injection of fluorophore-conjugated antibodies, hair on the ears of BALB/c mice was removed by a chemical depilatory agent. One day after hair removal, the mice were anesthetized, and the ears were injected intradermally on the ventral side with of an antibody cocktail consisting of phosphate buffered saline (PBS), Fc block ( ; BD Biosciences, San Diego, California), allophycocyanin (APC)-conjugated ( ; eBioscience, Inc., San Diego), and AlexaFluor488-conjugated ( ; Biolegend, San Diego). The first of these antibodies labels the two morphologically distinct yet related antigen-presenting cells—the LCs within the epidermis and iDCs in the dermis.10 The second labels the adhesion molecule PECAM, which is highly expressed on blood vessels and less intensely expressed on lymphatic vessels. Vessels reside only in the dermal layer, and labeling them contributes to the identification of the two DC populations. After to allow for the unlabeled antibody to clear, the mice were again anesthetized for imaging. In vivo imaging was performed using a custom inverted laser scanning confocal fluorescence microscope.13, 14 We have recently reported on the use of the antibody labeling technique in conjunction with the confocal imaging system to visualize the intratumor distribution of a photodynamic therapy sensitizer in vivo with respect to fluorophore-labeled CD31-positive vessels.15 In this study, to image the two DC populations and CD31-positive vessels, we used sequential two-color excitation of identical fields of view; APC was excited with a diode laser, and Alexa488 was excited with from an argon-ion laser. The APC and Alexa488 emissions were detected using a 647LP long-pass filter and a bandpass filter, respectively. The combination of a -diam pinhole and a , objective gave an optical section thickness of approximately , as determined by fluorescence edge response measurements.13 The images were acquired with a lateral resolution of . The mice were placed on the stage in the supine position so that the ventral side of the ear was facing downward for imaging. Confocal images were then acquired every , beginning at an initial depth of roughly from the surface. The images could then be analyzed individually or as a three-dimensional (3-D) volume. For all experiments, the appropriate isotype controls were used to rule out nonspecific staining. Confocal fluorescence images obtained in vivo show positive staining for both DC types, as evident by their unique morphologies at depths consistent with their localization in epidermal or dermal layers. Within the epidermis at a depth of approximately from the surface of the ear, an extensive mesh-network of densely packed LCs with characteristic long dendritic-like projections12 is shown in Fig. 1a . Optical sections acquired in the dermis at depths of approximately [Fig. 1b] show that the LC population is no longer present and that it is replaced by a stout, relatively sparse iDC population lacking long projections. Also evident in Fig. 1b and exclusive to the dermis is the extensive vasculature system, which is labeled with antibody and which serves as a useful biomarker to discriminate dermal from epidermal layers. We note that the iDCs tend to cluster tightly in the vicinity of the vasculature. Fig. 1In vivo confocal fluorescence images of cells in the mouse ear reveal two morphologically distinct cutaneous DC populations in superficial epidermal (a) and deeper dermal (b) layers of the tissue. In (b), dermal vessels are labeled with Alexa488-conjugated . (c) to (f) Ex vivo whole-mount histology confirms in vivo observations. Excised ears were split and processed; the epidermis was stained with (c) and (d) to confirm the identification and morphology of Langerhans cells. Image overlay (e) confirms the dual staining. (f) DCs in the dermis were stained with (red), and their distribution relative to -labeled vessels (green) is shown. These findings were confirmed in 10 and 20 mice for in vivo confocal and whole-mount imaging, respectively. The field of view in all images is . 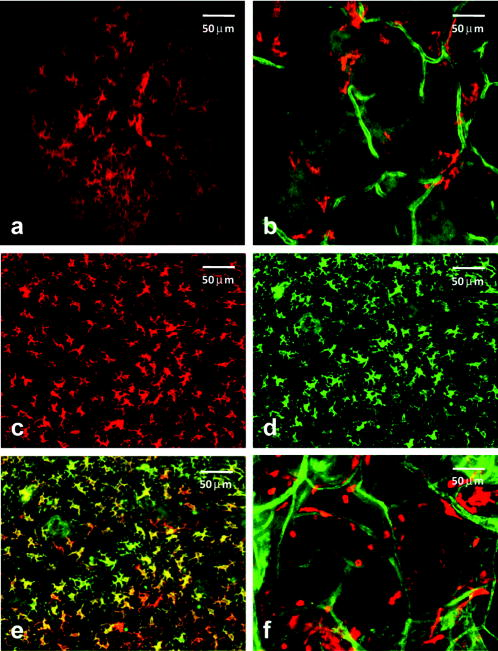 To confirm the identification of the DC populations imaged in vivo, we used whole-mount labeling of ex vivo tissues obtained from the dermal and epidermal layers of the ear and conventional fluorescence microscopy. Although both LCs and iDCs are MHC-II positive on their cell surfaces, only the LCs are positive for internal langerin, a C-type lectin unique to the cell and crucial for its development.16 Briefly, mice were sacrificed, ear hair was chemically removed, and ears were excised and split with the aid of forceps into dorsal and ventral halves. For dermal labeling, split ears were placed in of PBS with Fc Block , APC-conjugated , and phycoerythrin (PE)-conjugated and stained for at . To label the epidermis exclusively, additional tissue processing was required. Following ear splitting, halves were floated in ammonium thiocyanate at for to separate epidermal from dermal layers. The epidermal layer was then fixed in 2% paraformaldehyde at room temperature for to permeabilize the cells to enable labeling of internal langerin. Epidermal tissue was then incubated for with PE-conjugated and APC-conjugated . Positive staining of epidermal whole mounts with both [Fig. 1c] and internal [Fig. 1d] confirms the identification of these epidermal LCs. This critical confirmation can be performed only ex vivo, as cell permeabilization is not possible in vivo. The significant overlap between the images of the cell surface and internal markers for LCs is depicted in Fig. 1e. Single-color control experiments revealed no detectable cross talk between these two detection channels. The long cellular processes characteristic of these LCs are apparent in both the images obtained from the epidermal whole-mount preparation and from the superficial optical sections in vivo [Fig. 1a]. Ex vivo fluorescence imaging of the dermal layer [Fig. 1f] reveals vascular and DC cell morphology features similar to those observed in the images obtained from the deeper, dermal skin layer in vivo [Fig. 1b]. We have also implemented this technique to examine the tumor microenvironment in EMT6 mammary tumors grown intradermally in the ears of BALB/c mice. Tumors were initiated with an intradermal injection of EMT6 cells and grown to a diameter of approximately . cells and tumor blood vessels were labeled with APC-conjugated and Alexa488-conjugated , respectively, using intradermal injection of the antibody cocktail described earlier. As illustrated in Fig. 2 , in vivo confocal images acquired at two different depths after antibody administration revealed excellent, high-contrast staining of cells. Specifically, the signal levels from stained cells observed in Figs. 2a and 2c are at least four- to eight-fold higher than the adjacent background. Images acquired at below the surface [Figs. 2a and 2b] reveal a population of epidermal LCs morphologically similar to those seen in Figs. 1a and 1c. In comparison to the DCs imaged at a comparable depth in the normal ear [Figs. 1b and 1f], at a depth of in the tumor [Fig. 2c and 2d], the cells are more numerous, their distribution is more diffuse, and their morphologies suggest a mixed population that may include not only LCs and iDCs similar to the normal ear but also macrophages. The deeper image also shows the CD31-positive tumor vasculature. Fig. 2In vivo confocal fluorescence images of an EMT6 tumor after intradermal injection with fluorophore-conjugated and . (a) cells (red) visualized at a depth of in the tumor with morphology consistent with that of Langerhans cells. No CD31-positive vasculature is observed at this depth. (c) Positively stained cells (red) at a depth of in the tumor in the presence of a highly vascularized tumor microenvironment. Images (b) and (d) are expanded views of the region of interest indicated by the white box superimposed on (a) and (c), respectively. Imaging experiments were performed in 4 tumor-bearing mice, and the results were found to be reproducible. The field of view in images (a) and (c) is , while that in (b) and (d) is . 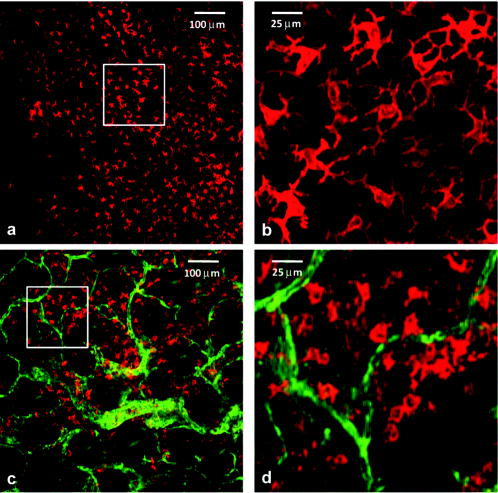 We note that the field of view of the in vivo images of Figs. 2a and 2c is and that antibody labeling of MHC-II and CD31 is efficient across the entire image following a single injection outside the imaging field. Thus, the antibody conjugates diffused laterally a distance of more than , which is much greater than the maximum depth at which quality images can be obtained with single-photon excited confocal fluorescence imaging. The method is therefore not limited by the depth of antibody diffusion but rather by attenuation of the excitation light. We note also that both DC populations are fully accessible to our imaging method in the mouse ear. This has been confirmed by successfully imaging CD31-positive vessels at depths of approximately , roughly beyond the layer where the iDCs are stratified in the normal ear (data not shown). The ability to image noninvasively specific cell types at high spatial resolution in vivo and the availability of a wide variety of fluorescently conjugated antibodies render this technique very attractive for many applications in immunology and tumor biology. A particular advantage of intradermal versus systemic injection4 is that it requires the use of significantly less antibody, and it can be performed with standard, commercially available antibody preparations with negligible toxicity as compared to that encountered from systemic administration. AcknowledgmentsThe authors acknowledge support from NIH Grant Nos. CA28332, UI9AI067733, and CA68409. ReferencesV. Ntziachristos,
“Fluorescence molecular imaging,”
Annu. Rev. Biomed. Eng., 8 1
–33
(2006). https://doi.org/10.1146/annurev.bioeng.8.061505.095831 1523-9829 Google Scholar
S. H. Yun,
G. J. Tearney,
B. J. Vakoc,
M. Shishkov,
W. Y. Oh,
A. E. Desjardins,
M. J. Suter,
R. C. Chan,
J. A. Evans,
I. K. Jang,
N. S. Nishioka,
J. F. de Boer, and
B. E. Bouma,
“Comprehensive volumetric optical microscopy in vivo,”
Nat. Med. (N.Y.), 12 1429
–1433
(2006). https://doi.org/10.1038/nm1450 1078-8956 Google Scholar
E. Brown,
T. McKee,
E. diTomaso,
A. Pluen,
B. Seed,
Y. Boucher, and
R. K. Jain,
“Dynamic imaging of collagen and its modulation in tumors in vivo using second-harmonic generation,”
Nat. Med. (N.Y.), 9 796
–801
(2003). https://doi.org/10.1038/nm879 1078-8956 Google Scholar
D. A. Sipkins,
X. Wei,
J. W. Wu,
J. M. Runnels,
D. Côté,
T. K. Means,
A. D. Luster,
D. T. Scadden, and
C. P. Lin,
“In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment,”
Nature (London), 435 969
–973
(2005). https://doi.org/10.1038/nature03703 0028-0836 Google Scholar
J. M. Runnels,
P. Zamiri,
J. A. Spencer,
I. Veilleux,
X. Wei,
A. Bogdanov, and
C. P. Lin,
“Imaging molecular expression on vascular endothelial cells by in vivo immunofluorescence microscopy,”
Mol. Imaging, 5 31
–40
(2006). 1535-3508 Google Scholar
S. Stoll,
J. Delon,
T. M. Brotz, and
R. N. Germain,
“Dynamic imaging of T cell–dendritic cell interactions in lymph nodes,”
Science, 296 1873
–1876
(2002). 0036-8075 Google Scholar
M. J. Miller,
S. H. Wei,
M. D. Cahalan, and
I. Parker,
“Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy,”
Proc. Natl. Acad. Sci. U.S.A., 100 2604
–2609
(2003). https://doi.org/10.1073/pnas.2628040100 0027-8424 Google Scholar
R. N. Germain,
M. J. Miller,
M. L. Dustin, and
M. C. Nussenzweig,
“Dynamic imaging of the immune system: progress, pitfalls and promise,”
Nat. Rev. Immun., 6 497
–507
(2006). 1474-1733 Google Scholar
C. Sumen,
T. R. Mempel,
I. B. Mazo, and
U. H. von Andrian,
“Intravital microscopy: visualizing immunity in context,”
Immunity, 21 315
–329
(2004). 1074-7613 Google Scholar
L. Mastropasqua,
M. Nubile,
M. Lanzini,
P. Carpineto,
M. Ciancaglini,
T. Pannellini,
M. Di Nicola, and
H. S. Dua,
“Epithelial dendritic cell distribution in normal and inflamed human cornea: in vivo confocal microscopy study,”
Am. J. Ophthalmol., 142 736
–744
(2006). 0002-9394 Google Scholar
S. A. Gerber,
V. Y. Rybalko,
C. E. Bigelow,
A. A. Lugade,
T. H. Foster,
J. G. Frelinger, and
E. M. Lord,
“Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth,”
Am. J. Pathol., 169 1739
–1752
(2006). 0002-9440 Google Scholar
J. Valladeau and
S. Saeland,
“Cutaneous dendritic cells,”
Semin Immunol., 17 273
–283
(2005). 1044-5323 Google Scholar
C. E. Bigelow,
C. J. Harkrider,
D. L. Conover,
T. H. Foster,
I. Georgakoudi,
S. Mitra,
M. G. Nichols, and
M. Rajadhyaksha,
“Retrofitted confocal laser scanner for a commercial inverted fluorescence microscope,”
Rev. Sci. Instrum., 72 3407
–3410
(2001). https://doi.org/10.1063/1.1382637 0034-6748 Google Scholar
C. E. Bigelow,
D. L. Conover, and
T. H. Foster,
“Confocal fluorescence spectroscopy and anisotropy imaging system,”
Opt. Lett., 28 695
–697
(2003). https://doi.org/10.1364/OL.28.000695 0146-9592 Google Scholar
S. Mitra and
T. H. Foster,
“In vivo confocal fluorescence imaging of the intratumor distribution of the photosensitizer mono-L-aspartylchlorin-e6,”
Neoplasia, 10 429
–438
(2008). 1522-8002 Google Scholar
J. Valladeau,
O. Ravel,
C. Dezutter-Dambuyant,
K. Moore,
M. Kleijmeer,
Y. Liu,
V. Duvert-Frances,
C. Vincent,
D. Schmitt,
J. Davoust,
C. Caux,
S. Lebecque, and
S. Saeland,
“Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules,”
Immunity, 12 71
–81
(2000). 1074-7613 Google Scholar
|

