|
|
1.IntroductionMicro-mechanical retention is established as the principal adhesion mechanism of dental adhesives.1 However, earlier dentin bonding studies suggest that specific functional monomers from adhesives can chemically interact with dental tissue components.2–4 The potential benefit of additional chemical interaction has been shown to contribute to adhesive performance,5,6 prolonging bond longevity of adhesive restorations.7,8 Detection of chemical bond between adhesives and dentin has shown to be a controversial topic in literature.9–11 As a consequence, several techniques have been used in order to provide a better understanding of the involved chemical processes like x-ray photoelectron spectroscopy (XPS),7 micromechanical tests,12 and especially those working in the infrared spectral region.2,13,14 It is well known that techniques based on infrared radiation are considered the most important route to access chemical bonding information of biological tissues.15–17 Among them, Fourier transform infrared photoacoustic spectroscopy (FTIR-PAS) has the special characteristics of allowing depth profile studies to detect the penetration and interaction of substances through biological tissues.9,18 It can be applied for measurements at clinical conditions because minimal sample preparation is needed. It is a non-destructive method and provides the inspection in opaque and highly scattering samples. This technique has been used before to investigate the occurrence of adhesive chemical bonds in dentin; exploring the finger print characteristics of the optical absorption bands in the infrared spectral region.9 Therefore, considering that there are no surfaces studies about the chemical interactions between human moist dentin blocks and etch-&-rinse adhesives in a clinical typical condition, the aim of this study was to apply FTIR-PAS spectroscopy to investigate the physicochemical structural surface changes of healthy human intact dentin after treatment with two commercial etch-&-rinse adhesives. 2.Materials and MethodsThree sound human premolars were collected from patients who needed extraction for orthodontic reasons in conformity with an informed consent protocol reviewed and approved by the local ethics committee in human research. From the central part of the buccal and lingual faces, two specimens () were cut off per tooth using a low-speed Isomet 1000 diamond saw. Therefore, a total of six paired specimens () were produced. Through the use of paired specimens both adhesives could be tested on the same dentin substrate, minimizing the influence of dentin composition on the obtained results. Control sample homogeneity was proved by the calculation of dentin untreated surface mineral:matrix ratio ( the ratio of the integrated areas of the phosphate, and , in relation to the amide I peak). These ratios were submitted to Shapiro-Wilk normality test (SPSS 10.0 statistical package), and were represented by means of standard deviation. According to the use of different adhesive systems: A— (Heraeus Kulzer, Hanau, Germany); B—Single Bond 2 (3M-ESPE, St Paul, MN, USA), the fragments were divided into two groups (). The compositions of the adhesives are shown in Table 1. They present distinct functional monomers, as: A) 2-hydroxyethylmethacrylate (HEMA) and 4-methacryloxyethyl trimellitate anhydrate (4-META), and B) HEMA. Table 1Composition and manufacture specification of the studied adhesives.
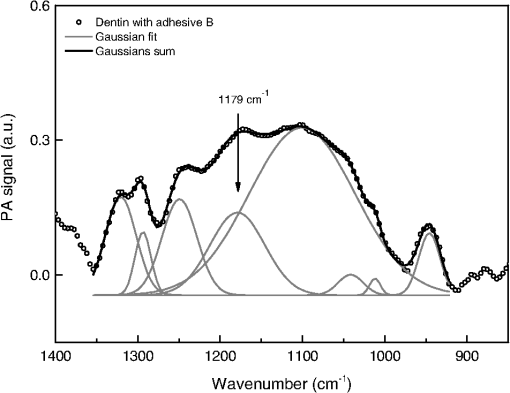
Data provided by the manufacturers. All the specimens were acid-etched for 15 s with 37% phosphoric acid, water rinsed for 5 s and blotted-dry, leaving the dentin surface moist. The adhesives were applied following the manufacturer’s instructions. There were some differences in the treatments of each group: Group A received application of three adhesive layers and was polymerized for 20 s using a blue LED light source of , while Group B received an application of two layers and was polymerized for 10 s. The final thickness of each film was calculated taking into account the initial adhesive volume and the sample superficial area. This was made neglecting both volume change during polymerization and the amount of interpenetrated adhesive in the dentin hybrid layer. Then, the values for groups A and B were found to be about 3 and 2 µm, respectively. To verify that, the samples were inspected with electron scanning microscopy (MEV) technique, model Shimadzu-SS-550 superscan system. It was noted that the films did not present constant thicknesses along the dentin, varying in an interval of about 1 to 4 µm. The thermal lens (TL) technique, described elsewhere,19 was used to measure the thermal diffusivity values of the adhesive films. To do so, the films were deposited in a microscope slide and polymerized as described above. After polymerization, it was gently removed and the TL measurements performed. Sequential analysis mode was used as an experimental approach, so that the chemical modifications of the same dentin surface were verified by the FTIR-PAS technique before and after the procedures of acid conditioning and adhesives application. Moreover, the spectra of adhesives alone were taken after polymerization of 10 µl of each liquid, according to the manufacturer’s instructions. For each measurement, either the selected dentin surface or the adhesive sample was placed in the sample holder and inserted into a MTEC 300 photoacoustic cell. Before sealing the cell, the chamber was purged with helium gas to avoid spectral interference by loosely bound water.20,21 In addition, the use of helium gas in the PAS cell improved the signal/noise ratio significantly. All spectra were recorded in the rapid scan mode on a FTIR-PAS spectrophotometer, Varian 7000, between 500 and at a mirror speed of (10 kHz) and a resolution of . Five hundred to 1000 scans were collected in order to increase the signal-to-noise ratio, and all spectra were compared to a carbon black reference provided by MTEC. To specify the technique ability to provide the depth of analysis in our experimental condition, it is important to consider that in the rapid scan mode, the FTIR-PAS method provides the thermal diffusion length (cm) as , in which D is the sample thermal diffusivity (), is the mirror velocity (), and the incident radiation wavelength (cm). This parameter is the dimension over which the thermal wave decays to of its original amplitude and has been used in the analysis as approximately the sampling depth, where the PAS signal is generated. In our experimental condition, the sample can be approached as comprised of two layers with a hybrid interface, resulting in effective values for the thermal diffusivity. It depends on each values, according to the incident radiation wavelength. For the dentin in the tubular direction, ,22 while for the adhesives, the TL measurements performed in this work provided similar values, . Since the exciting radiation was incident on the adhesive side of the samples, we estimate using the adhesives thermal diffusivity values. Thus, using , we calculate, for example, the penetration depth at () to be higher than 5.0 µm. Analyses of adhesives-treated spectra were done through spectra baseline and subtraction.23 For each group, the etched-dentin spectra were subtracted from the adhesive-treated dentin spectra obtained from the same specimen surface, and the spectra of the difference were compared with that of the original adhesives. Subtracted spectra Gaussian fittings were made in order to help the identification of absorption bands. Furthermore, some absorption bands modified after the adhesives treatment were also fitted by Gaussian functions in order to better identify the spectra alterations. It is well known that the absorption bands in the infrared permit the chemical bonding modifications to be traced, expressed by peak shifting and/or new peak formations. Due to the qualitative characteristic of the data resulting from the FTIR-PAS, statistical analysis was not carried out in this study. 3.ResultsSpectra of the six control dentin surface showed homogeneity in organic and inorganic composition (). The etching with 37% phosphoric acid resulted in a decalcification pattern; presenting increased relative intensities and/or better resolved absorption bands of amide groups centered around (amide I), (amide II), and (amide III), and decreased intensities of absorption bands at (carbonate stretching vibrations—), (orthophosphates stretching vibrations—) and (carbonate stretching vibrations—), as shown in Fig. 1. Because of the dentin spectra modifications caused by the etching procedure, the acid-conditioning spectra were considered as negative control for the analysis of adhesives interactions. Fig. 1FTIR-PAS spectra: demineralization pattern verified through the comparison between acid-etched dentin and control dentin surface. 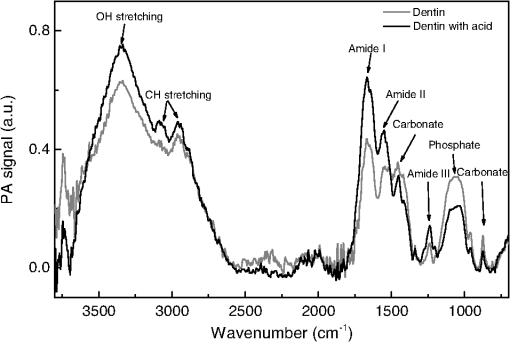 The spectra subtraction evidenced that some dentin peaks were modified after the application of both adhesives. In Figs. 2(a) and 3(a), in the interval between 3800 and , a slight peak decrease of the OH stretching (OH-bonding) occurred at as a result of adhesive treatment, and also a significant reduction of the band at (symmetric C-H stretching) took place when comparing spectra of original adhesives with that of the dentin-treated adhesives. Furthermore, the ratio of bands of the subtracted spectra decreased for adhesive A and increased for adhesive B. Fig. 2FTIR-PAS spectra: adhesive A, dentin with acid, dentin treated with adhesive A and subtracted spectra: (a) spectra range between 3600 and showing modifications due to adhesive interaction; (b) spectra1800 to range, indicating a orthophosphate optical absorption band (1040 to ) reduction because of chemical reaction with adhesive. 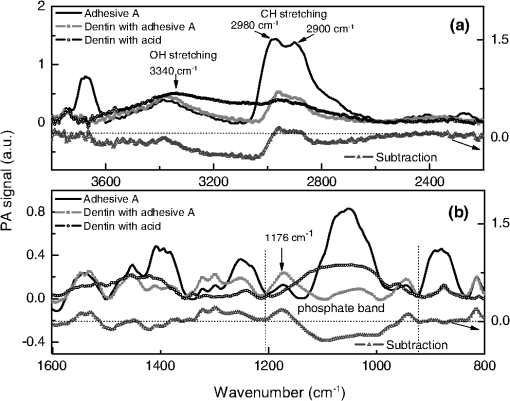 Fig. 3FTIR-PAS spectra: adhesive B, dentin with acid, dentin treated with adhesive B and subtracted spectra: (a) spectra wavelength between 3600 and showing modifications due to adhesive interaction; (b) spectra 1800 to range, suggesting the occurrence of chemical bonding of adhesive attributed to the calcium/phosphate ester complexes formation. 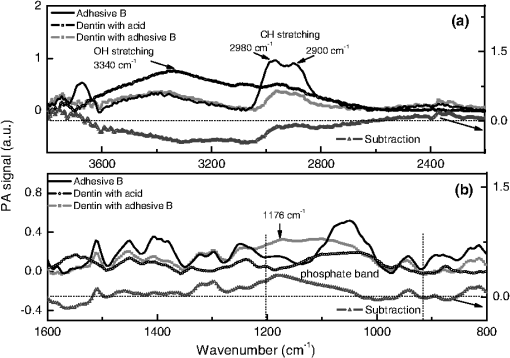 In addition, differences in spectra subtraction at the interval from 1600 to can be seen in Figs. 2(b) and 3(b). The subtracted curve for group A, in Fig. 2(b), shows both a relevant decrease of orthophosphate absorption band (1040 to ), and an increase of the peak related to phosphoric group (). Nevertheless, in Fig. 3(b), another pattern of modification can be noticed in the dentin treated with adhesive B and on the subtracted spectra. These alterations suggest the formation of a calcium-phosphate-ester complex (1270 to ), as observed before by Penel et al.24 Gaussian fittings were made at the interval of 1400 to of the dentin-treated adhesives to identify the compound absorption bands, as shown in Fig. 4. The band deconvolution of adhesive B-treated spectrum provides the identification of a better resolved band at . 4.DiscussionTo the best of our knowledge, this is the first analysis reporting chemical interactions of etch-&-rinse adhesives with human intact and moist dentin treated with commercial adhesive system. After demineralization with 37% acid etching (Fig. 1), mineral components dissolution was evidenced from the reduction of the orthophosphate-carbonate peak, and collagen exposure was verified by the increased intensities of the amide groups absorption bands. The spectra alterations after treatment with adhesives A and B indicated their chemical interactions with dentin (Figs. 2Fig. 3–4). Detecting true chemical bonding information of the interface depends on the exclusive analysis of the bonded layer.25 Acid conditioning of sound dentin can result in a demineralization depth around 6 to 8 µm,26,27 while the hybrid layer thickness is believed to be approximately 6 µm.28 Since the FTIR-PAS average depth profile in the experimental condition of this work was around 5 µm, and the adhesive surface layer thicknesses smaller than 3 and 2 µm, for adhesives A and B, respectively, this technique characterized the structure, including the chemical interactions at the interface region, between the dentin and the adhesive. Both adhesives selected for this study have HEMA as a functional monomer. However, only adhesive A contains the monomer 4-META. Previous studies have suggested that HEMA reacts with the collagenous fraction of dentin,11,29 and also that it is associated with the formation of calcium/phosphate ester complexes in the hybrid layer.3,24 In the case of 4-META, its ability to chemically bond to calcium hydroxyapatite has been described.7,30 According to the manufacturers, adhesive A also contains poly(methacrylic-oligoacrylic) acid and glutaral, and adhesive B methacrylate functionalized copolymer of acrylic and polyitaconic acids. These compounds are also active and may bond to the dentin as well. These reinforce the complexity to determine the type of adhesive chemical bonding to the dentin. The modifications found after the adhesive treatment at spectra between 3800 and were presented for both adhesives [Figs. 2(a) and 3(a)], evidencing their capacity to chemically interact with the collagenous fraction of dentin. Moreover, despite the fact it is also present in the adhesive spectra, the increase of the absorption band around (phosphoric group ) for group A [Fig. 2(b)] and the occurrence of a wide band at this spectral region in group B [Figs. 3(b) and 4] (probably revealing the formation of a calcium/phosphate ester complexes) are further indications of adhesives bonding to the inorganic dentin matrix. The reduction of the OH stretching absorption bands around is the evidence of functional group formation via transesterification. This reaction occurs when the ester portion of HEMA reacts with the protein hydroxyl group, resulting in the formation of ester and alcohol and the consumption of .11 The peak at on the adhesives spectra can be attributed to symmetric C-H stretching. Thus, the decrease of this band, when the original adhesives and dentin-treated adhesives were compared, may be assigned to the reaction of part of the adhesive molecules to the available protein sites. In addition, the occurrence of an absorption band around (phosphoric group ) may also be an indication of chemical interaction between ester of the adhesive and calcium and phosphate dentin molecules.3,24 Functional monomers of adhesive system A appears to have more chemical affinity to the dentin inorganic matrix (calcium ion) than adhesive B since its spectra did not show evidence of complex formation, presenting only an increase of the peak (phosphoric group ). Moreover, the significant reduction of the phosphate absorption band (1040 to ) after treatment with adhesive A [Fig. 2(a)] indicates the chemical reaction of its functional monomer to dentin. This modification suggests the occurrence of ionic binding between carboxylic groups of adhesive to Hap ions;31 a mechanism in which those carboxylic groups replace and extract the phosphate ions of the dentin substrate.7 Although the chemical interaction is dependent on the adhesive composition,32 the third layer of adhesive A applied on dentin could have increased the adhesive performance of this system. The use of sound human dental tissue in blocks was advantageous because the organic-mineral matrix and water junction of the dentin tissue remained with its natural structure.4,32,33 However, in vitro studies may be limited to simulate the clinical conditions. The dentin specimens used were non-vital; therefore, the dentinal fluid flow produced by the intrapulpal pressure was not present in our experimental approach. In order to minimize this limitation, the dentin blocks were kept in saline solution and gently dried before starting the treatment. Another disadvantage found in these analyses consisted of bands overlapping in the 1785 to spectral region.13 Because of the interaction of the neighboring bands, it was preferable to disregard the modifications of the amide group absorption bands after adhesive treatment. However, in terms of the tentative to detect the presence of chemical bonding, the use of PAS-FTIR technique permitted the evaluation of these non-homogeneous samples in a procedure in which little sample preparation was required. Especially, the method was able to make depth profile inspection down to about 5 µm from the sample surface, including the region where adhesive dentin interaction was supposed to take place. To conclude, according to FTIR-PAS investigation, the spectra alterations suggested that both adhesives presented chemical bonding to dentin, a study performed using a typical clinical treatment. The results indicated chemical evidence of the transesterification reaction through adhesive molecules and collagen hydroxyl group, chemical interaction of adhesives to inorganic portion of dentin forming calcium/phosphate ester complexes and, also ionic bonding between carboxylic groups of adhesives and calcium hydroxyapatite. The results reinforced that the adhesive’s chemical interaction with dentin depends on their compositions. Since adhesive restorations have limited durability in the mouth and chemical-bonding adhesives are expected to extend bond longevity, the observations on this work may help with the actual goal of the dental material industry for improvements of adhesives that chemically bond to dentin available sites. AcknowledgmentsThe authors gratefully acknowledge the Brazilian National Agencies for Scientific and Technological Development CNPq, CAPES, FINEP, and Fundação Araucária, for supporting this investigation. A preliminary study was presented at the 2010 meeting of the International and American Associations for Dental Research, Barcelona, Spain. ReferencesJ. Perdigao,
“Dentin bonding-variables related to the clinical situation and the substrate treatment,”
Dent. Mater., 26
(2), E24
–E37
(2010). http://dx.doi.org/10.1016/j.dental.2009.11.149 DEMAEP 0109-5641 Google Scholar
B. P. Fu et al.,
“Evidence of chemical bonding to hydroxyapatite by phosphoric acid esters,”
Biomaterials, 26
(25), 5104
–5110
(2005). http://dx.doi.org/10.1016/j.biomaterials.2005.01.035 BIMADU 0142-9612 Google Scholar
M. A. Latta,
“Shear bond strength and physicochemical interactions of XP Bond,”
J. Adhes. Dent., 9
(Suppl. 2), 245
–248
(2007). JADEFV 1461-5185 Google Scholar
B. Van Meerbeek et al.,
“Relationship between bond-strength tests and clinical outcomes,”
Dent. Mater., 26
(2), E100
–E121
(2010). http://dx.doi.org/10.1016/j.dental.2009.11.148 DEMAEP 0109-5641 Google Scholar
J. De Munck et al.,
“A critical review of the durability of adhesion to tooth tissue: methods and results,”
J. Dent. Res., 84
(2), 118
–132
(2005). http://dx.doi.org/10.1177/154405910508400204 JDREAF 0022-0345 Google Scholar
S. Inoue et al.,
“Hydrolytic stability of self-etch adhesives bonded to dentin,”
J. Dent. Res., 84
(12), 1160
–1164
(2005). http://dx.doi.org/10.1177/154405910508401213 JDREAF 0022-0345 Google Scholar
K. Nagakane et al.,
“Analysis of chemical interaction of 4-MET with hydroxyapatite using XPS,”
Dent. Mater. J., 25
(4), 645
–649
(2006). http://dx.doi.org/10.4012/dmj.25.645 DMJOD5 Google Scholar
K. L. Van Landuyt et al.,
“Systematic review of the chemical composition of contemporary dental adhesives,”
Biomaterials, 28
(26), 3757
–3785
(2007). http://dx.doi.org/10.1016/j.biomaterials.2007.04.044 BIMADU 0142-9612 Google Scholar
P. Spencer et al.,
“Chemical characterization of the dentin adhesive interface by Fourier transform infrared photoacoustic spectroscopy,”
Dent. Mater., 8
(1), 10
–15
(1992). http://dx.doi.org/10.1016/0109-5641(92)90046-F DEMAEP 0109-5641 Google Scholar
B. Van Meerbeek et al.,
“Chemical characterization of the resin-dentin interface by micro-Raman spectroscopy,”
J. Dent. Res., 72
(10), 1423
–1428
(1993). http://dx.doi.org/10.1177/00220345930720101201 JDREAF 0022-0345 Google Scholar
M. Di Renzo et al.,
“Adhesion to mineralized tissue: bonding to human dentin,”
Prog. Surf. Sci., 50
(1–4), 407
–418
(1995). http://dx.doi.org/10.1016/0079-6816(95)00072-0 PSSFBP 0079-6816 Google Scholar
P. Spencer et al.,
“Adhesive/dentin interface: the weak link in the composite restoration,”
Ann. Biomed. Eng., 38
(6), 1989
–2003
(2010). http://dx.doi.org/10.1007/s10439-010-9969-6 ABMECF 0090-6964 Google Scholar
Y. Wang, X. Yao and R. Parthasarathy,
“Characterization of interfacial chemistry of adhesive/dentin bond using FTIR chemical imaging with univariate and multivariate data processing,”
J. Biomed. Mater. Res. Part A, 91A
(1), 251
–262
(2009). http://dx.doi.org/10.1002/jbm.a.v91a:1 1549-3296 Google Scholar
Y. Wong, X. Yao and P. Spencer,
“Micro-Raman imaging analysis of monomer/mineral distribution in intertubular region of adhesive/dentin interfaces,”
J. Biomed. Opt., 11
(2), 024005
(2006). http://dx.doi.org/10.1117/1.2187992 JBOPFO 1083-3668 Google Scholar
A. Deshmukh et al.,
“Raman spectroscopy of normal oral buccal mucosa tissues: study on intact and incised biopsies,”
J. Biomed. Opt., 16
(12), 127004
(2011). http://dx.doi.org/10.1117/1.3659680 JBOPFO 1083-3668 Google Scholar
G.-B. Jung et al.,
“Effect of cross-linking with riboflavin and ultraviolet A on the chemical bonds and ultrastructure of human sclera,”
J. Biomed. Opt., 16
(12), 125004
(2011). http://dx.doi.org/10.1117/1.3662458 JBOPFO 1083-3668 Google Scholar
G. J. Zhang, L. Senak and D. J. Moore,
“Measuring changes in chemistry, composition, and molecular structure within hair fibers by infrared and Raman spectroscopic imaging,”
J. Biomed. Opt., 16
(5), 056009
(2011). http://dx.doi.org/10.1117/1.3580286 JBOPFO 1083-3668 Google Scholar
M. L. Baesso, R. D. Snook and J. J. Andrew,
“Fourier transform infrared photoacoustic spectroscopy to study the penetration of substances through skin,”
J. Phys. IV, 4
(C7), 449
–451
(1994). http://dx.doi.org/10.1051/jp4:19947104 JPICEI 1155-4339 Google Scholar
R. D. Snook, R. D. Lowe and M. L. Baesso,
“Photothermal spectrometry for membrane and interfacial region studies,”
Analyst, 123
(4), 587
–593
(1998). http://dx.doi.org/10.1039/a706757g ANLYAG 0365-4885 Google Scholar
M. G. Sowa and H. H. Mantsch,
“FT-IR photoacoustic depth profiling spectroscopy of enamel,”
Calcif. Tissue Int., 54
(6), 481
–485
(1994). http://dx.doi.org/10.1007/BF00334328 CTINDZ 0171-967X Google Scholar
M. Di Renzo et al.,
“A photoacoustic FTIRS study of the chemical modifications of human dentin surfaces, I: demineralization,”
Biomaterials, 22
(8), 787
–792
(2001). http://dx.doi.org/10.1016/S0142-9612(00)00240-4 BIMADU 0142-9612 Google Scholar
M. F. de Magalhaes et al.,
“Measurement of thermophysical properties of human dentin: effect of open porosity,”
J. Dentistry, 36
(8), 588
–594
(2008). http://dx.doi.org/10.1016/j.jdent.2008.04.006 JDENAB 0300-5712 Google Scholar
G. Eliades, G. Vougiouklakis and G. Palaghias,
“Heterogeneous distribution of single-bottle adhesive monomers in the resin-dentin interdiffusion zone,”
Dent. Mater., 17
(4), 277
–283
(2001). http://dx.doi.org/10.1016/S0109-5641(00)00082-8 DEMAEP 0109-5641 Google Scholar
G. Penel et al.,
“Qualitative and quantitative investigation of calcium phosphate of biological interest by Raman micro-spectrometry,”
Recent Res. Dev. Appl. Spectrosc., 2 137
–146
(1999). Google Scholar
L. Breschi et al.,
“Dental adhesion review: Aging and stability of the bonded interface,”
Dent. Mater., 24
(1), 90
–101
(2008). http://dx.doi.org/10.1016/j.dental.2007.02.009 DEMAEP 0109-5641 Google Scholar
Y. Wang and X. Yao,
“Morphological/chemical imaging of demineralized dentin layer in its natural, wet state,”
Dent. Mater., 26 433
–442
(2010). http://dx.doi.org/10.1016/j.dental.2010.01.002 DEMAEP 0109-5641 Google Scholar
P. Spencer et al.,
“Interfacial chemistry of the dentin/adhesive bond,”
J. Dent. Res., 79
(7), 1458
–1463
(2000). http://dx.doi.org/10.1177/00220345000790070501 JDREAF 0022-0345 Google Scholar
Y. Wang and P. Spencer,
“Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy,”
J. Biomed. Mater. Res., 59
(1), 46
–55
(2002). http://dx.doi.org/10.1002/(ISSN)1097-4636 JBMRBG 0021-9304 Google Scholar
J. Xu et al.,
“An FT-Raman spectroscopic investigation of dentin and collagen surfaces modified by 2-hydroxyethylmethacrylate,”
J. Dent. Res., 76
(1), 596
–601
(1997). http://dx.doi.org/10.1177/00220345970760011101 JDREAF 0022-0345 Google Scholar
Y. Yoshida et al.,
“Evidence of chemical bonding at biomaterial-hard tissue interfaces,”
J. Dent. Res., 79
(2), 709
–714
(2000). http://dx.doi.org/10.1177/00220345000790020301 JDREAF 0022-0345 Google Scholar
Y. Yoshida et al.,
“Comparative study on adhesive performance of functional monomers,”
J. Dent. Res., 83
(6), 454
–458
(2004). http://dx.doi.org/10.1177/154405910408300604 JDREAF 0022-0345 Google Scholar
K. L. Van Landuyt et al.,
“Influence of the chemical structure of functional monomers on their adhesive performance,”
J. Dent. Res., 87
(8), 757
–761
(2008). http://dx.doi.org/10.1177/154405910808700804 JDREAF 0022-0345 Google Scholar
L. Bachmann et al.,
“Infrared absorption bands of enamel and dentin tissues from human and bovine teeth,”
Appl. Spectros. Rev., 38
(1), 1
–14
(2003). http://dx.doi.org/10.1081/ASR-120017479 APSRBB 0570-4928 Google Scholar
|