|
|
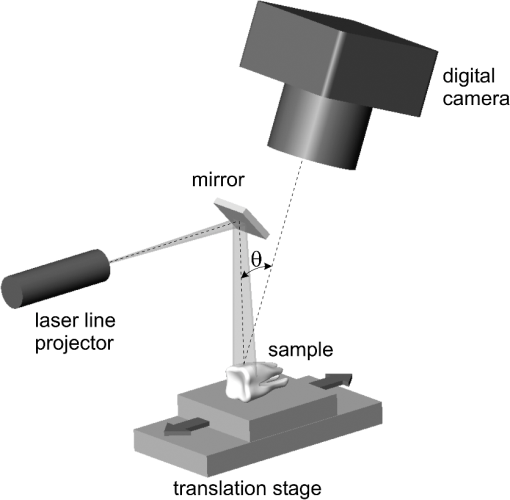
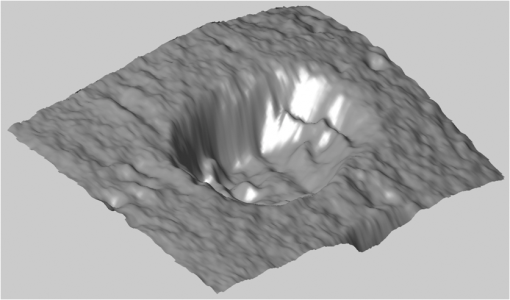
1.IntroductionErbium lasers have become established tools for fast and safe ablation of hard tissues.1–4 This is due to the highly efficient absorption of erbium laser energy in water molecules, which have a strong absorption band centered near the 3-μm emission wavelength of erbium lasers. Two erbium wavelengths have been developed for using clinically on hard dental tissues. These include the Er:YAG (2.94 μm) and the Er,Cr:YSGG (2.78 μm), which by many scientific accounts have very similar properties. These two wavelengths make up the erbium family of dental lasers. When comparing the wavelengths of the erbium lasers at room temperature, the Er:YAG laser’s wavelength of 2.94 μm matches the absorption peak of water, while the absorption coefficient in water for the 2.78 μm is approximately three times lower. Fast subsurface expansion of laser-heated water trapped within the interstitial structure of hard dental tissue was proposed to be the primary mechanism by which the erbium lasers performed ablation.2–7 It was also discovered very early on that local cooling by adding a water film to the tooth surface significantly improves the process of erbium laser ablation.8–10 All published studies show that adding water, either in the form of spray or in a thin layer, prevents discoloration. It also improves ablation efficiency (AE) in enamel by preventing a reduction or even stalling without further tissue removal when a series of laser pulses is delivered to the tissue.8–18 As shown by measurements of the optoacoustic signal for a series of consecutive Er:YAG laser pulses, the optoacoustic energy is larger for initial pulses and is reduced toward the end of the pulse sequence.11,12 With water applied in-between pulses, no reduction in the optoacoustic signal is observed. This was attributed to tissue desiccation where, without the applied water, the sequence of laser pulses dries out the hard tissue surface, which results in a reduced efficiency of laser ablation and consequently in a reduced optoacoustic signal. Tissue desiccation of laser-irradiated hard tissue surfaces has also been observed using infrared spectroscopy.19,20 Another mechanism for erbium laser ablation of hard dental tissue, different from subsurface micro-explosions, was also considered as an explanation for the observed beneficial effect of adding water.18,21–23 It was suggested that a “hydrokinetic effect”21 could be obtained by introducing atomized water droplets via a nozzle into the space between the laser handpiece and the tooth surface. As highly absorbing to the laser radiation, the water droplets in the spray were considered to vaporize explosively, producing a substantial mechanical ablation to the tooth surface upon impact. The hydrokinetic effect was described as a new phenomenon, which does not require the presence of water film on the tooth surface to obtain highly efficient cutting. Instead, water particles are directed in a manner to receive energy from laser radiation, and subsequently impart disruptive forces to the target.23 On the other hand, an independent experimental study24 brought the conclusion that if the proposed hydrokinetic effect exists, it is not effective on hard materials, which are void of water, and it does not contribute any significant degree in the ablation of dental enamel. A recent study25 pointed out that the actual mechanism of interaction among the water spray or water layer, the laser irradiation, and the hard tissues is not yet clearly understood and is somewhat controversial. In order to shed more light on this topic, we carried out a systematic comparison study of Er:YAG and Er,Cr:YSGG laser ablation under three experimental conditions. In one of the experimental setups, standard dental laser water spray was applied continuously to the tooth. In another experimental setup, the water port of the water spray was turned off during laser radiation, while the air was allowed to blow continuously on the tooth surface in order to prevent any water pooling. Different experimental water cooling conditions were chosen, in order to distinguish between (a) conditions originally proposed in Ref. 8, where only covering the tooth surface with a water layer prior to or during the laser beam is required, (b) conditions originally proposed in Ref. 10, where water is added prior to or during the laser pulse in order to have water present in surface pores or being chemically bound to the material at the surface, and (c) conditions required by Refs. 22 and 23, where additional water mist must be present above the tooth surface during laser radiation. In case the hydrokinetic effect exists, the AE should improve when additional water mist is present above the tooth surface during laser radiation. A highly accurate and repeatable methodology for the measurement of ablated volumes in teeth was used in the study. This method is based on a laser triangulation principle and had been successfully applied in previous hard-tissue laser ablation studies.26–29 2.Materials and MethodsThe Er:YAG laser used was a Fidelis Plus III (manufactured by Fotona, Slovenia, European Union), and the Er,Cr:YSGG system was a WaterLase iPlus (manufactured by Biolase, Irvine, CA). The Er:YAG laser system was fitted with either a tipless handpiece (R02, with 800-μm beam spotsize in focus) or a fiber-tip handpiece (R14, with Varian, 940-μm diameter fiber tip).30 The Er,Cr:YSGG system was equipped with a fiber-tip handpiece (“Gold,” with MGG6, 600-μm diameter fiber tip). Unless otherwise stated, water-spray cooling as provided by the laser systems was used in the experiments. The experiments were conducted on randomly chosen extracted premolar and molar teeth, which were stored in a physiological saline solution immediately following extraction. Before each ablation experiment, the tooth was positioned to have its surface perpendicular to the laser beam and to be at a focal distance of the laser beam (10 mm from the handpiece exit window when the R02 handpiece was used). For the fiber-tip handpieces, the tooth was positioned to have its surface perpendicular to the handpiece fiber tip and to be at a fixed distance of 0.4 mm with regard to the fiber tip using a micrometer stage. The fixed distance of 0.4 mm for both laser wavelengths was chosen, because previous published studies have indicated that the fiber-tip AE depends slightly on the distance between the fiber tip and the tooth surface and is largest at about 0.4 mm.12,13 Measurements were made on enamel, which covers the crown of the tooth, and on cementum, which covers the root of the tooth. Enamel and cementum were chosen in order to evaluate the effects of Er:YAG laser radiation on hard tissues at both extremes, i.e., in enamel which has a very low intrinsic water content of 3%weight and in cementum with a relatively high intrinsic water content of 22% weight.31 Note that dentin contains 12% weight of water, bone contains approximately 22% weight of water, and soft tissue contains 70% weight of water. Each ablation cavity was made with 10 consecutive laser pulses delivered to the same spot on the same tooth. The volume of the ablated cavity following 10 pulses was then measured using the laser triangulation method. Following the same procedure, cavities were made on three different spots on the same tooth and repeated on three different teeth. Four cavities instead of three cavities were made on one of the three teeth. Each ablation-volume data point, thus, represents an average obtained from 10 cavities, each made with 10 consecutive pulses (altogether 100 pulses). Since the AE varies significantly among tooth samples, special care was taken that each set of comparison measurements was made on the same group of tooth samples. The goal of our measurements was to study the ablation dynamics during each laser pulse delivered to the tooth. However, since the detection sensitivity of the laser profilometer used in our study was not high enough to reliably measure the ablated volumes created by single laser pulses, we measured volumes as obtained with 10 consecutive pulses at a slow repetition rate of 0.2 Hz (i.e., with a pulse period of ). This provided us with sufficient time to modify water cooling conditions in between laser pulses. Single-pulse laser energies were measured with an external energy meter at the handpiece output. Unless otherwise stated, 200 mJ of laser pulse energy were used on cementum and 300 mJ on enamel. In the case of the Er:YAG system, the slow repetition rate of 0.2 Hz was achieved by controlling an internal shutter. In the case of the Er,Cr:YSGG system, it was left intact, and the 0.2 Hz were achieved by providing an appropriate external footswitch signal to the system. A specialized measurement apparatus26–29 was used to determine the very small volumes of ablated material. Figure 1 shows a schematic of the apparatus. The measured surface is illuminated by a diode laser beam formed into a light plane. The bright laser beam is visible on the illuminated surface. An image of the illuminated surface is acquired by a digital camera and transferred to a computer, where it is analyzed to determine a profile of the illuminated surface. By moving the sample using a linear translation stage, a series of about 2000 surface profiles is acquired and converted to a three-dimensional model of the surface, which consists of about 1 million measured points. A custom software “Volume_analyser”26–29 is then used to analyze the model surface of the ablated crater and to determine the volume of the ablated material. A detailed description of the apparatus and the underlying method is presented in Ref. 27. The design of the system enables highly accurate and repeatable measurements as well as the facility for photographic recording and visual comparison (see Fig. 2). The measurements were designed to determine the AE, i.e., the ablated volume per pulse energy (in ), for five experimental conditions:
Fig. 3“Spray” conditions: coaxially directed, built-in water/air spray turned on continuously; (a) Tipless and (b) fiber-tip handpiece configurations are shown. 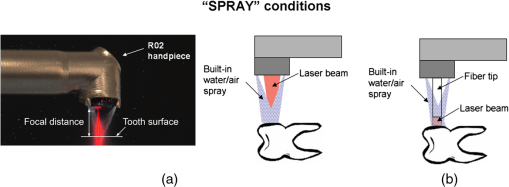 Fig. 4Water/spray and laser-pulse sequence under “pores” experimental conditions. Only tipless handpiece configuration is shown; however, the same pulse sequence was applied also with fiber-tip handpieces. For the Er,Cr:YSGG “pores” experiments, an external water and air device was used. 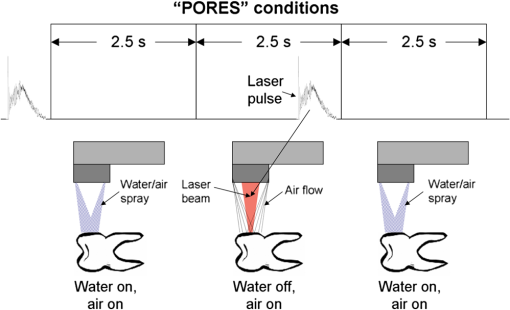 Fig. 6“Dry+brush” ablation conditions. No water or air was applied to the tooth. The ablated surface of the tooth was brushed with a toothbrush after each laser pulse, in order to remove ablation debris. 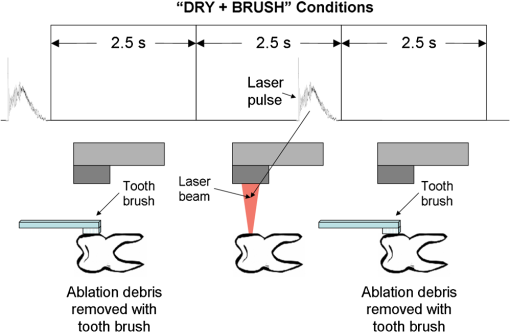 The above experimental conditions are represented together in Table 1. Table 1Description of the experimental conditions used in the study.
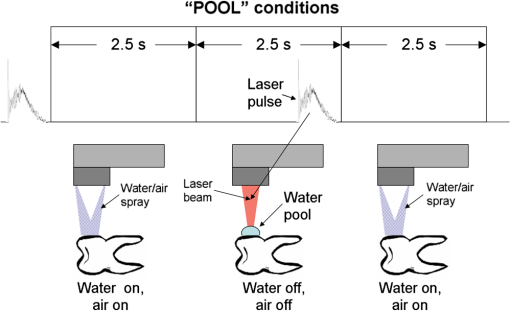
The “spray” experimental setup was assumed to correspond to the conditions as required to obtain the hydrokinetic effect.22,23 The goal of the “pores” experimental setup was to establish conditions as required by Ref. 10. In some of the experiments, a third “pool” experimental setup was used, during which both water and air were turned off 2.5 s prior the laser pulse leaving a water pool on the tooth surface prior to the onset of laser radiation (Fig. 5). In the fourth, the “dry” experimental setup, water/air spray was turned off for the entire time. In the fifth, the “dry+brush” experimental setup, the ablated laser tooth surface was brushed with a standard toothbrush following each laser pulse, in order to at least partially remove the remaining ablation deposits (Fig. 6). The measured data was analyzed using the statistics tools of the Gnumeric Spreadsheet 1.10.16 computer program and the MATLAB Statistics Toolbox. The reported results have been calculated assuming the significance level of 5% () unless otherwise explicitly noted. Five standard Fidelis Er:YAG pulse duration modes, SSP, MSP, SP, LP, and VLP, and the H mode of the WaterLase Er,Cr:YSGG were used in the experiments. At 200 mJ of laser-pulse energy, the full width at 10% maximum pulse durations as obtained from the measured averaged pulse shapes were as follows: H (290 μs), SSP (120 μs), MSP (170 μs), SP (290 μs), LP (430 μs), and VLP (750 μs).32 Output pulses from both lasers were nonsymmetric with their full-width-half-maximum closer to the beginning than to the end of the pulse, as required for the hydrokinetic effect.22,23 3.ResultsFigures 7Fig. 8Fig. 9Fig. 10Fig. 11 through 12 present the key experimental results: measured AE (in ) obtained under various processing conditions. In the charts, the column height represents the mean value of the sample (with repeated measurements). The error bars represent the sample standard deviation (SD). It is important to note in the visual assessment of the charts that the estimates of the standard error of the mean (SEM) are: , meaning that the length of SEM error bars is in fact only about 32% the length of the plotted SD error bars. Fig. 7Measured AE in cementum obtained with a tipless R02 Er:YAG handpiece under “spray” and “pores” conditions for different pulse durations. The analysis of variance (ANOVA’s) calculated value for the effect of water cooling conditions: , is above the critical (crit. ), which indicates that the difference between “spray” and “pores” conditions is statistically significant irrespective of the level of the other independent variable (laser-pulse duration). (The symbol represents the posteriori probability that the obtained result occurred by chance.) 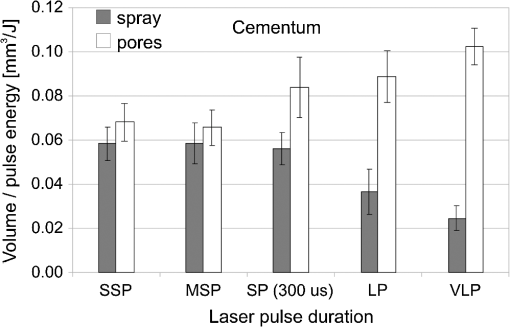 Fig. 8Measured AE in enamel obtained with a tipless R02 Er:YAG handpiece under “spray” and “pores” conditions for different pulse durations. The ANOVA’s calculated value for the effect of water cooling conditions: , is above the critical (crit. ), which indicates that the difference between “spray” and “pores” conditions is statistically significant irrespective of the level of the other independent variable (laser-pulse duration). 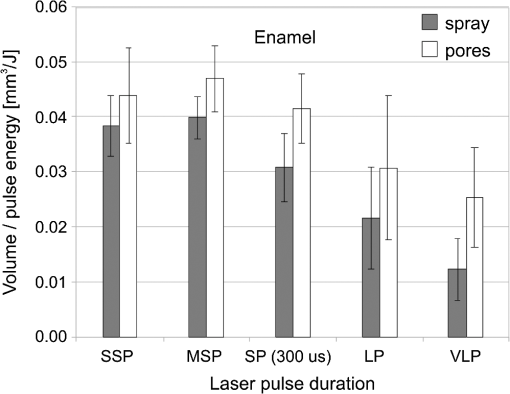 Fig. 9Measured AE in cementum and in enamel obtained with a tipless R02 Er:YAG handpiece under “pool,” “,” “,” “,” “pores,” and “dry” experimental conditions. For all measurements, the pulse duration was SSP. The ANOVA’s calculated value for the effect of water cooling conditions: , is above the critical (crit. ), which indicates that the difference between water cooling conditions is statistically significant irrespective of the level of the other independent variable (dental tissue type). Pairwise comparison of ablation efficiencies using either “” or “” and “pores” conditions did not show statistically significant differences: , and , (crit. ). 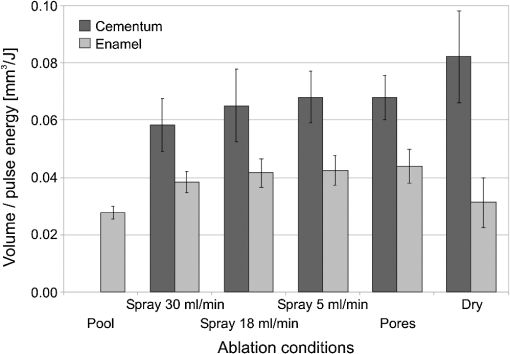 Fig. 10Measured AE in enamel and in cementum obtained for both wavelengths under the “spray” and “pores” conditions. The ANOVA’s calculated values for the effect of water cooling conditions: , and , for enamel and cementum, respectively, are above the critical (crit. ), which indicates that the difference between “spray” and “pores” conditions is statistically significant irrespective of the wavelength. 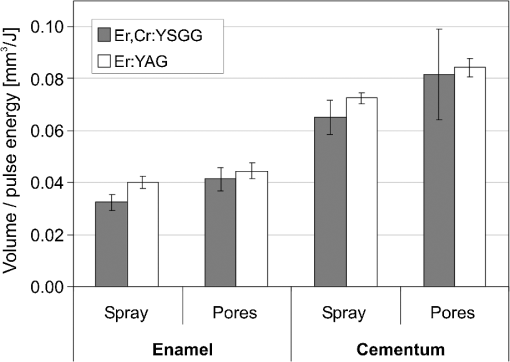 Fig. 11Measured AE in cementum and in enamel obtained with a tipless R02 Er:YAG handpiece under “dry+brush” and “dry” experimental conditions. The pulse duration was SSP, and pulse repetition was 0.2 Hz. The ANOVA’s calculated value for the effect of water cooling conditions: , is above the critical (crit. ), which indicates that the difference between ablation conditions is statistically significant irrespective of the level of the other independent variable (dental tissue type). 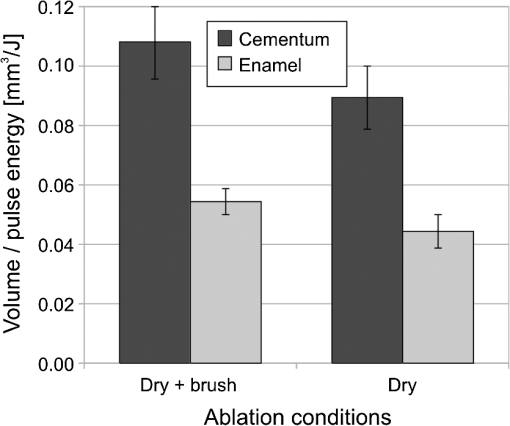 Fig. 12Measured AE in enamel obtained with a tipless R02 Er:YAG handpiece under “spray,” “dry,” and “dry+brush” experimental conditions. The pulse duration was SSP. The ANOVA’s calculated value for the effect of water cooling conditions: , is above the critical (crit. ), which indicates that the difference between ablation conditions is statistically significant. Pairwise comparison of ablation efficiencies using “spray” and “dry+brush” conditions did not show statistically significant differences: , (crit. ). 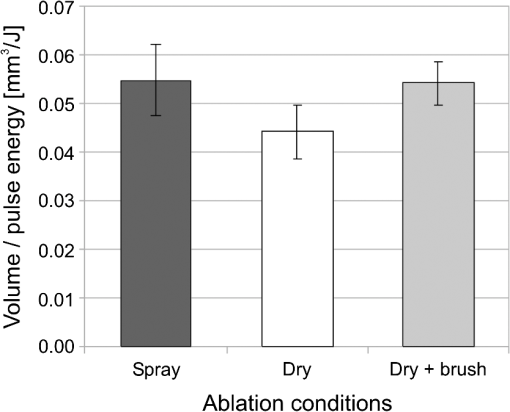 In order to assess the statistical significance of the observations, we conducted two-way analysis of variances (ANOVAs) on the data presented in Figs. 7 through 11, which take AE as the dependent variable and water cooling condition and either laser pulse duration or dental tissue type or laser wavelength as the independent variables. One-way ANOVA was conducted on the data presented in Fig. 12, which takes AE as the dependent variable and water cooling condition as the independent variable. Figures 7 and 8 show the measured AE (in ) obtained with a tipless handpiece R02 in cementum and enamel, respectively, for different pulse durations under “spray” and “pores” experimental conditions. The water-spray level was set to the starting recommended setting by the Er:YAG laser manufacturer of . This setting is within the range of water-spray levels used in previous studies ( in Ref. 33 and in Ref. 17). For both enamel and cementum, “pores” conditions resulted in a statistically significant higher AE compared with “spray” conditions, irrespective of whether pulse duration was shorter or longer than 300 μs. In order to check whether there may be an optimal level of water spray for achieving the hydrokinetic effect, we also measured the AE in cementum and enamel at the SSP pulse duration for three different water-spray levels: 30, 18, and (see Fig. 9). The results show that the AE is negatively affected by the increased level of water spray and is largest when no spray () is applied, i.e., under “pores” conditions. The lowest AE was observed under “pool” conditions. For comparison, measurements were also made under “dry” conditions. We also measured the dependence of AE of the Er,Cr:YSGG laser wavelength (H mode; laser energy of 220 mJ) under “spray” and “pores” conditions, and compared it with that of the Er:YAG (SSP mode; laser energy of 200 mJ in cementum and 300 mJ in enamel) (see Fig. 10). For this set of measurements, both laser types were equipped with the corresponding fiber-tip handpieces. For “spray” conditions, the Er,Cr:YSGG water and air spray settings were as recommended by the laser manufacturer for Restorative Class I preparations. The Er:YAG spray level was . For both, enamel and cementum, “pores” conditions resulted in a statistically significant higher AE compared with “spray” conditions, irrespective of whether Er:YAG or Er,Cr:YSGG wavelength was used. In the final part of the study, we measured the influence of condensed mineral phases from the ablation plume (which are deposited along the crater walls) on the stalling of the AE under “dry” conditions. In this setup, we carried out comparison ablation measurements in cementum and enamel under “dry” and “dry+brush” ablation conditions (see Fig. 11). As can be seen from Fig. 11, brushing of the tooth surface following each laser pulse removes loosely bound deposits from the ablation area and significantly improves the AE in both cementum and enamel. In cementum, tooth brushing improves the AE over that obtained under “dry” conditions. From Fig. 9, which shows that ablation under “dry” conditions is faster than under “spray” conditions, we can conclude that the AE under “dry+brush” conditions is also much higher than the efficiency under “spray” conditions. For comparison, we also repeated the AE measurements in enamel under “spray” conditions for the same group of tooth samples. Figure 12 shows the obtained AE in enamel under “spray” conditions, together with the ablation efficiencies under “dry” and “dry+brush” conditions. The AE in enamel under “dry+brush” conditions is, within experimental error, comparable with that under “spray” conditions, which indicates that the dehydrated and thermally modified ablation deposits are the major cause for ablation stalling in both cementum and enamel. Since relatively mild brushing for just a few seconds was applied in our experiments, it is conceivable that deposits were not completely removed and that some of the more strongly bound minerals may still have remained on the tooth surface following the brushing. It is possible that with more aggressive brushing, the obtained AE in enamel would be, as in cementum, not only comparable, but also even higher than when using water spray. 4.Discussion4.1.Influence of Water Cooling ConditionsOur measurements have demonstrated that any water that is present above the tooth surface in a form of water-spray droplets or on the surface of the tooth in a form of a water layer or pool reduces the AE of erbium lasers. No beneficial effect of water spray and, therefore, no hydrokinetic effect were observed, regardless of the laser wavelength used. Water spray leads only to a reduction of AE, since part of the incident laser energy gets partially used to create a “vapor tunnel” required for the radiation to reach the tooth surface.34,35–37 Similarly for drilling teeth with a standard dental bur, water cooling during erbium laser treatments is required in order to prevent desiccation and temperature buildup within the tooth. However, water cooling does not contribute to the ablation process itself. Instead, it actually reduces the efficiency of optical drilling in teeth by absorbing part of the incident laser energy. Further confirmation that spray functions only as an absorber and not as an enhancer of the AE can be obtained by observing that on Figs. 7 and 8 the difference between the AE under “spray” and “pores” conditions is larger for longer pulse durations. This can be explained by noting that during longer pulse durations more water is delivered to the tooth, and therefore, more laser energy is required to sustain the vapor tunnel. Since the amount of water delivered to the tooth during a laser pulse depends linearly on laser-pulse duration, the relative decrease in AE should also increase linearly with laser-pulse duration. If we define the relative decrease in AE due to the presence of spray as and take into account the AE data shown in Figs. 7 and 8, we obtain, as expected, an approximately linear dependence of on the pulse duration and consequently on the amount of water delivered during the duration of each laser pulse (see Fig. 13).Fig. 13Relative reduction in AE, , in enamel and in cementum as a function of pulse duration. The reduction, as obtained from the data shown in Figs. 10 and 11, is normalized to a laser-pulse energy of 200 mJ for both dental tissues. 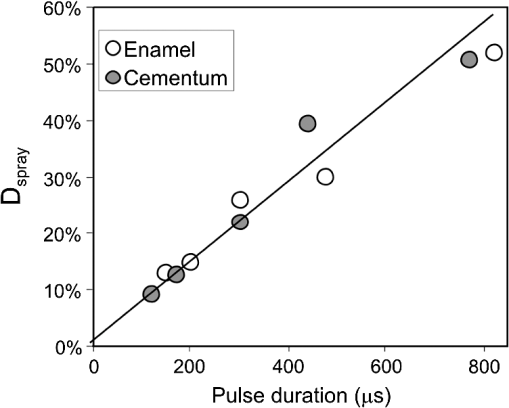 The fact that the data for both dental tissues shown in Fig. 13 can be fitted very well with the same linear function that originates at zero value for zero pulse duration (i.e., at the limit of no spray water delivered to the tooth during laser radiation), represents a very strong additional confirmation that water-spray functions only as an absorber of laser radiation and not as an ablative agent. Figure 13 also demonstrates that no additional effect occurs at pulse durations below 300 μs. It is also important to note that the AE in cementum is highest under “dry” conditions (see Fig. 9). Any application of external water, i.e., even adding water to enter tooth surface pores, negatively affects the AE. This is in agreement with our conclusion above that the ablation effect can be attributed solely to a fast subsurface expansion of the laser-heated water trapped within the interstitial structure of the hard dental tissues and not to the proposed disruptive forces of the accelerated particles of externally added water above the tooth surface. As our measurements show, the observed reduction in the AE for dry enamel, as opposed to when the enamel is wetted between pulses (see Fig. 9), can be attributed mainly to the collection of loosely attached melted or recondensed minerals arising from the preceding laser pulses. This layer of material is water deficient and is more resistant to explosive ablation and removal with subsequent Er:YAG laser pulses. By adding water to the tooth surface, and thus, by rehydrating the porous superficial layer, laser pulses are able to cleanse the surface and to restart the ablation process. A similar effect can be achieved also by mechanical means. When in our experiments, a toothbrush was used to brush off any loosely attached minerals following each laser pulse, the AE in enamel can be recovered to approximately the same level, and in cementum, even improved above the AE achieved under water-spray conditions (see Figs. 11 and 12). As shown by measurements of the optoacoustic signal for a series of consecutive Er:YAG laser pulses, the optoacoustic energy is larger for initial pulses and is reduced toward the end of the pulse sequence.11,12 With water applied, no reduction in the optoacoustic signal was observed. As discussed above, this is due to the fact that without the applied water, the sequence of laser pulses results in the collection of dehydrated minerals on the hard tissue surface, which leads to a reduced efficiency of laser ablation and consequently in a reduced optoacoustic signal. The desiccation effect, as detected through the reduction of the optoacoustic signal, is largest for enamel, where after five laser pulses, the optoacoustic signal falls to approximately 10% of the initial signal. Under the same conditions, the signal in dentin falls only to 80% of the initial signal, which indicates that the effects of desiccation are less pronounced in hard tissues with higher water content. This explains why in our measurements the cementum with relatively high water content showed a further increase in the AE when water cooling was completely eliminated. We believe, however, that if a single-pulse AE could be accurately measured, this increased AE under “dry” conditions would also be observed for enamel, as evidenced by our measurements under “dry+brush” conditions. Since typical measured rates of water diffusion in hard tissues are slow, some authors concluded that tissue rehydration is not available at higher laser repetition rates.17,19 However, it must be taken into account that only a very thin layer of tissue needs to be rehydrated to prevent stalling of laser ablation. For thin layers of enamel, the effective water-diffusion coefficient, , has been measured to be .38 The diffusion time can then be calculated from38 where is the diffusion distance. The diffusion time required for water to diffuse deep into the enamel is approximately , which is sufficiently short for most laser repetition rates. Even more importantly, as shown by our experiments, laser-modified porous superficial layer of enamel with expectedly a higher diffusion coefficient, , plays a decisive role in the ablation process. Rehydration of dental hard tissue under spray conditions can, therefore, take place also at higher laser repetition rates. In a study by Meister et al.,17 erbium laser ablation rates were measured depending on whether hard tissues were untreated or dehydrated prior to the experiment. At of external water spray, no statistically significant difference in Er:YAG and Er,Cr:YSGG laser AE was observed between water-containing and dehydrated enamel or dentin. Since laser repetition rates up to 20 Hz were used, this is in agreement with the conclusion that the diffusion of externally supplied water can be sufficiently fast to rehydrate thin superficial layers of hard dental tissues even at high laser repetition rates.Based on the above, the hard-tissue laser ablation process can be described as follows: In the first phase of the laser ablation process, rapid heating of the tissue within the laser penetration depth leads to subsurface heating of water that is confined in the sound hard-tissue matrix.39 The interstitial confinement of the subsurface water leads to subsurface pressures of hundreds of bars7 and to material failure and explosive removal of the overlying hard tissue in the form of a plume. However, not all of the ablated material gets removed away from the ablation area. Some loosely attached desiccated melted or recondensed mineral remains on the surface and can lead to a reduction or even stalling of ablation by subsequent laser pulses. As our measurements show, ablation stalling can be prevented to a large degree by mechanically brushing away the loosely attached material, and thus making the ablation hole free of desiccated material before each subsequent laser pulse arrives. Alternatively, this step can be replaced by adding water to the ablated area. Externally added water rehydrates the ablation deposits and the loosely bound tissue and enables the initial part of the subsequent laser pulse to cleanse the ablation hole with any ablation residue. The mechanism for the removal of the ablation residue is the same as for the removal of sound tissue. Water that is confined within the superficial layer is rapidly heated, which leads to explosive evaporation. Adding water and subsequent laser removal, thus, serve a similar function as brushing off the deposits with a toothbrush. After the ablation hole has been cleansed, the remaining major part of the laser pulse can proceed with the explosive ablation of the underlying sound hard-tissue matrix. Following the laser pulse, the ablation area is again covered by the loosely attached and recondensed mineral residue, and the process of surface cleansing can be repeated again. Measurements of the optoacoustic signal for a series of consecutive Er:YAG laser pulses show that under “dry” conditions in enamel, most of the ablation stalling occurs already within the first 10 pulses, while under “spray” conditions, no ablation stalling is detected.11,12 Since in the present study, the ablation volume was measured following 10 consecutive pulses, and the measured volume under “pores” conditions was even larger than under “spray” conditions, it is our conclusion that wetting of the ablation cavity in-between laser pulses, while preventing any water pooling, is more than sufficient to prevent ablation stalling in enamel. This is confirmed by Fig. 14, which shows the measured ablation cavity depths in enamel following 10, 20, and 30 consecutive Er:YAG pulses under “dry,” “spray ,” “spray ,” and “pores” experimental conditions. The “pores” experimental conditions not only prevent ablation stalling, but are also most efficient for ablation. Fig. 14Measured depths of ablation cavities following 10, 20, and 30 consecutive Er:YAG laser pulses (300 mJ, LP pulse duration, R02 handpiece) under “pores,” “water spray” (15 and ), and “dry” conditions. Each data point represents an average depth of 10 ablation cavities, as measured using a focusing optical microscope technique. 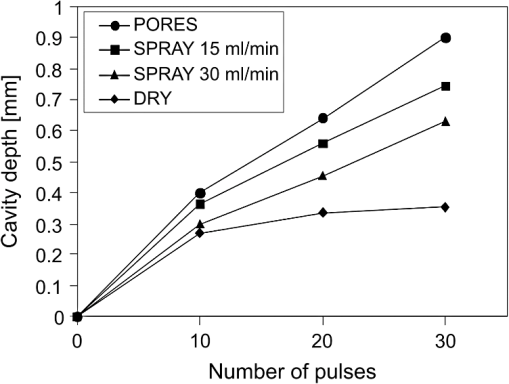 The effectiveness of the removal of the loose residual material under “pores” conditions can be seen also from Fig. 15, which shows craters in cementum under different water cooling experimental conditions. Fig. 15Ablation cavities in cementum following 10, 200 mJ, LP Er:YAG laser pulses under “spray” (a), “pores” (b), and “dry” (c) conditions. 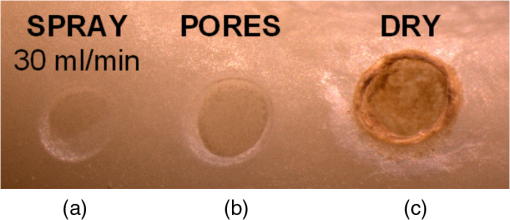 As can be seen from Fig. 15, under “dry” conditions, the ablation residue does not get removed by the subsequent laser pulses, which results in over-heating and charcoaling of the residual material and the surrounding tissue. On the other hand, no charcoaling is observed either under “spray” or “pores” conditions, which indicates that residual material is effectively removed under both conditions. It is important to note that similar lack of charcoaling was also observed under “dry+brush” conditions. Results of our study also indicate that any mechanism, such as mechanical brushing, that removes the loosely attached and recondensed mineral residue during the time in-between laser pulses will also minimize ablation stalling and improve AE. For example, we observed that some of the loose residue could be washed away and consequently AE improved by simply letting the water spray flow over the ablation area for 1 min in-between laser pulses. It is important to note that the experimental conditions in this study were designed with the specific goal to distinguish between the effects of tissue rehydration and the hypothesized ablative hydrokinetic effect. In a clinical setup where laser pulses are delivered at a high laser repetition rates, water cooling is required not only to prevent ablation stalling, but also to prevent temperature buildup, similar to when mechanical drills are used. Only wetting or mechanically brushing the teeth in-between pulses would therefore not be recommended for clinical applications. Results of our study do suggest, however, that stopping the water spray during the short laser pulse, or delivering water spray from the side directly to the tooth surface, and without intersecting the laser beam above the tooth, would improve erbium laser-AE. 4.2.Influence of Pulse DurationThe observed influence of laser-pulse duration on the AE under “pores” conditions (Figs. 7 and 8) can be attributed to the interplay of two counter-acting mechanisms: thermal diffusion and ablation plume shielding.40 On one hand, at long pulse durations, the energy has more time to escape from the ablated volume and so more heat is diffused into the surrounding tissue, which leads to a decreased AE at longer pulse durations. On the other hand, the density of the ejected debris that is formed above the surface is for the same laser pulse energy lower at longer pulse durations, and so there is less interference of the debris with the incident laser beam. Majaron et al.40 have shown that for the laser fluence of used in our experiment, the ablation in enamel is for pulse durations above approximately 300 μs, limited mainly by heat diffusion. For dentin, which has a lower ablation threshold due to the higher water content, debris screening was found for pulse durations below 1 ms to become dominant for pulse already at fluences above approximately . These findings are in agreement with our measurements. The AE in enamel is observed to decrease toward longer pulse durations, while in cementum, with even higher water content than dentin, AE improves with longer pulse durations. 4.3.Influence of Laser WavelengthWhen comparing the wavelengths of the erbium lasers, the Er:YAG laser’s wavelength of 2.94 μm matches the absorption peak of water, while the absorption coefficient in water for the 2.78 μm wavelength is significantly (three times) lower.41,42 In hard dental tissues, the absorption in the mineral content (hydroxyapatite) must also be taken into account, particularly in enamel, with its much lower water content. However, although hydroxyapatite absorbs more strongly the Er,Cr:YSGG wavelength,43 it is the stronger water absorption at the Er:YAG wavelength that plays the dominant role in dental laser ablation. Namely, the absorption coefficient in enamel of the Er:YAG wavelength has been measured to be approximately two times higher than that of the Er,Cr:YSGG.5,19 The difference in the absorption coefficients leads to a difference in the penetration depths of the two erbium laser wavelengths in dental tissues. In comparison with the Er:YAG, the Er,Cr:YSGG laser wavelength penetrates depending on the water content in the tissue, approximately two to three times deeper into the tissue.5,42 This difference is potentially important as it influences the volume of the directly illuminated tissue that needs to be rapidly heated to ablative temperatures by the laser light (direct heating), before the absorbed energy is spread out into the surrounding tissue by the process of thermal diffusion (indirect heating).40,44 Therefore, the higher the penetration depth, the larger the volume of directly heated tissue that needs to be rapidly heated up and the longer the time required to reach the ablation temperature. For effective ablation with minimal thermal side effects, it is important that the ablation process takes place over a short time, so that very little heat is transferred to the surrounding tissue.3,40 Based on this consideration alone, the Er:YAG laser wavelength is at an advantage and should exhibit a larger AE than Er,Cr:YSGG. However, since the spectroscopy literature indicates that the absorption peak of water decreases and shifts toward shorter wavelengths for increasing temperature,41,42 it has been suggested that in the ablation process, the absorption of the Er:YAG laser should decrease and the absorption of Er,Cr:YSGG should increase, perhaps even above that of Er:YAG.45 For this reason, it has been theorized that the AE of the Er,Cr:YSGG should actually be higher compared with that of the Er:YAG.45 On the other hand, some researchers have concluded that under high laser intensities, the dynamic optical properties of water should lead to a higher AE of the Er:YAG laser wavelength.42 Our measurements show the AE of the Er:YAG wavelength to be higher than that of Er,Cr:YSGG under all experimental conditions. This observation suggests that if there is any effect of the absorption shift on the AE, it is not large enough to make the AE of Er,Cr:YSGG lasers higher than that of Er:YAG lasers. It is also possible that the difference in the pulse durations of the two laser types may have over-shadowed any effects of the absorption shift. As our measurements have also shown, the AE is reduced when laser energy is delivered to the tooth over a longer time (see Figs. 7 and 8). Figure 16 shows the measured temporal development of the cumulative output laser energy over the duration of the laser pulse for both laser types used in the experiments. Fig. 16Temporal development of the cumulative output laser energy for Er,Cr:YSGG (H mode) and Er:YAG (SSP mode) at 200 mJ of total (100%) cumulative energy. 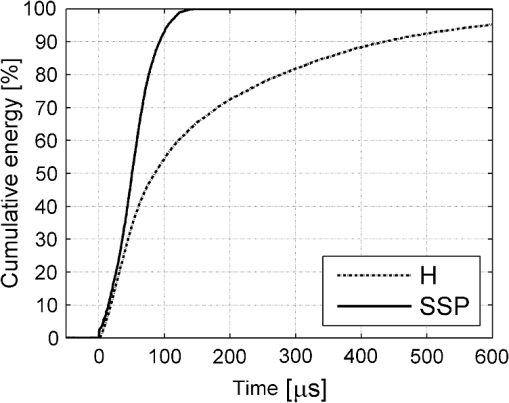 As can be seen from Fig. 16, the delivery of the Er,Cr:YSGG laser energy is significantly slower than that of the Er:YAG laser. Note that after the Er:YAG laser pulse has already ended, there still remains approximately 35% of undelivered energy within the Er,Cr:YSGG laser pulse. 5.ConclusionsA “hydrokinetic” effect in laser ablation of hard dental tissues has been hypothesized by Refs. 22 and 23 as an alternative mechanism to the now generally accepted mechanism of “subsurface water expansion.” In this study, precise measurements of Er:YAG and Er,Cr:YSGG laser AE in human enamel and cementum were performed under different ablation conditions, in order to verify the existence of the hydrokinetic effect. No evidence could be found for either of the erbium wavelengths that the alleged hydrokinetic effect is existent at all. In enamel, the highest AE, with clean cavities and no signs of thermal damage, was observed under the “pores” conditions when the superficial dental tissue was rehydrated only during the time in-between laser pulses. However, when in addition to the rehydration in-between the pulses, the pulsed laser beam was allowed to intersect with water-spray particles above the tooth (“spray” conditions), or with a water layer on the tooth surface (“pool” conditions), resulted in a reduction of AE. The carried-out measurements thus confirm that the application of water spray with the goal to provide a hydrokinetic effect results in a negative influence on ablation efficiency rather than improving the same, as would be anticipated by any assumed hydrokinetic effect. Our findings are in agreement with the now generally accepted mechanism of laser ablation of biological tissues, which is based on the fast subsurface expansion of laser-heated water trapped within the interstitial structure of the hard dental tissues. Our measurements also show that the well-known phenomenon of ablation stalling during a series of consecutive laser pulses can primarily be attributed to the blocking of laser light by the loosely bound and recondensed desiccated minerals that collect on the tooth surface during and following laser ablation. In addition to the prevention of the tooth bulk temperature buildup, a positive function of the water spray that is typically used with erbium dental lasers is to rehydrate these minerals, and thus sustaining the subsurface expansion ablation process. A negative side effect of using a continuous water spray is that the AE gets reduced due to the laser light being partially absorbed in water-spray particles above the tooth and in the collected water pool on the tooth surface. It is worth noting that the “new laser–matter interaction concept” (i.e., the hydrokinetic effect) was originally proposed to explain the observed lack of thermal effects and ablation stalling when water spray was used in conjunction with erbium laser radiation.18,22,35 As our study shows, both phenomena are observed under “pores” and “dry+brush” conditions and can thus be explained using the standard subsurface water expansion ablation model. Our measurements also do not confirm the hypothesis that Er,Cr:YSGG lasers cut faster in hard dental tissues than Er:YAG lasers tissues because of the water absorption shift during laser ablation. In agreement with previously published studies,26,28,46,47 the Er:YAG laser was found to be more effective for cutting human hard tissue than Er,Cr:YSGG. AcknowledgmentsThis work was partially financed by the European Regional Development Fund and the Slovenian Government. ReferencesJ. T. Walsh,
“Pulsed laser ablation of tissue: analysis of the removal process and tissue healing,”
Massachusetts Institute of Technology,
(1988). Google Scholar
R. HibstU. Keller,
“Experimental studies of the application of the Er:YAG laser on dental hard substances: I. Measurement of the ablation rate,”
Lasers Surg. Med., 9
(4), 338
–344
(1989). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
J. T. WalshT. J. FlotteT. F. Deutsch,
“Er:YAG laser ablation of tissues: effect of pulse duration, and tissue type on thermal damage,”
Lasers Surg. Med., 9
(4), 314
–326
(1989). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
U. KellerR. Hibst,
“Experimental studies of the application of the Er:YAG laser on dental hard substances: II. Light microscopic, and SEM investigations,”
Lasers Surg. Med., 9
(4), 345
–351
(1989). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
R. Hibst,
“Lasers for caries removal, and cavity preparation: state of the art, and future directions,”
J. Oral Laser Appl., 2
(4), 203
–211
(2002). 1473-7809 Google Scholar
B. MajaronP. PlestenjakM. Lukač,
“Thermo-mechanical laser ablation of soft tissue: modeling the micro-explosions,”
Appl. Phys. B, 69
(1), 71
–80
(1999). http://dx.doi.org/10.1007/s003400050772 APBOEM 0946-2171 Google Scholar
W. Sekaet al.,
“Laser ablation of hard dental tissues from explosive ablation to plasma mediated ablation,”
Proc. SPIE, 2672 144
–158
(1996). http://dx.doi.org/10.1117/12.238763 PSISDG 0277-786X Google Scholar
J. A. Hokeet al.,
“Erbium:YAG (2.94 um) laser effects on dental tissues,”
J. Laser Appl., 2
(3–4), 61
–65
(1990). http://dx.doi.org/10.2351/1.4745270 JLAPEN 1042-346X Google Scholar
L. Gradet al.,
“Optoacoustic studies of Er:YAG laser ablation in hard dental tissues,”
Proc. SPIE, 2128 456
–465
(1994). http://dx.doi.org/10.1117/12.184931 PSISDG 0277-786X Google Scholar
M. Lukačet al.,
“Optoacoustic effects during Er:YAG laser ablation in hard dental tissues,”
Proc. SPIE, 2327 93
–100
(1994). http://dx.doi.org/10.1117/12.197595 PSISDG 0277-786X Google Scholar
M. Lukačet al.,
“Interaction thresholds in Er:YAG laser ablation of organic tissue,”
Proc. SPIE, 2623 129
–138
(1995). http://dx.doi.org/10.1117/12.230324 PSISDG 0277-786X Google Scholar
S. R. Visuriet al.,
“Shear strength of composite bonded to Er:YAG laser-prepared dentin,”
J. Dent. Res., 75
(1), 599
–605
(1996). http://dx.doi.org/10.1177/00220345960750011401 JDREAF 0022-0345 Google Scholar
S. R. VisuriJ. T. WalshH. A. Wigdor,
“Erbium laser ablation of dental hard tissue: effect of water cooling,”
Lasers Surg. Med., 18
(3), 294
–300
(1996). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
D. Šušterčičet al.,
“Saturation effects in ablation of hard dental tissues by Er:YAG,”
Proc. SPIE, 2080 55
–59
(1993). http://dx.doi.org/10.1117/12.166189 PSISDG 0277-786X Google Scholar
J. Meisteret al.,
“Influence of water content in dental enamel and dentin on ablation with erbium YAG and erbium YSGG lasers,”
J. Biomed. Opt., 11
(3), 034030
(2006). http://dx.doi.org/10.1117/1.2204028 JBOPFO 1083-3668 Google Scholar
I. M. RizoiuL. G. DeShazer,
“New laser-matter interaction concept to enhance hard tissue cutting efficiency,”
Proc. SPIE, 2134A 309
–317
(1994). PSISDG 0277-786X Google Scholar
D. Friedet al.,
“Mechanism of water augmentation during IR laser ablation of dental enamel,”
Lasers Surg. Med., 31
(3), 186
–193
(2002). http://dx.doi.org/10.1002/(ISSN)1096-9101 LSMEDI 0196-8092 Google Scholar
L. Bachmannet al.,
“Changes in chemical composition, and collagen structure of dentine tissue after erbium laser irradiation,”
Spectrochim. Acta A, 61
(11–12), 2634
–2639
(2005). http://dx.doi.org/10.1016/j.saa.2004.09.026 SAMCAS 1386-1425 Google Scholar
L. R. EversoleI. RizoiuA. I. Kimmel,
“Pulpal response to cavity preparation by an erbium,chromium:YSGG laser powered by a hydrokinetic system,”
J. Am. Dent. Assoc., 128
(8), 1099
–1106
(1997). Google Scholar
R. J. FreibergC. Cozean,
“Pulsed erbium laser ablation of hard dental tissue: the effects of atomized water spray vs water surface film,”
Proc. SPIE, 4610 74
–84
(2002). http://dx.doi.org/10.1117/12.469306 PSISDG 0277-786X Google Scholar
X. Zhanget al.,
“Influence of water layer thickness on hard tissue ablation with pulsed laser,”
J. Biomed. Opt., 17
(3), 038003
(2012). http://dx.doi.org/10.1117/1.JBO.17.3.038003 JBOPFO 1083-3668 Google Scholar
T. PerhavecJ. Diaci,
“Comparison of Er:YAG, and Er,Cr:YSGG dental lasers,”
J. Oral Laser Appl., 8
(2), 87
–94
(2008). 1473-7809 Google Scholar
T. Perhavecet al.,
“A method for rapid measurement of laser ablation rate of hard dental tissue,”
Opt. Laser Technol., 41
(4), 397
–402
(2009). http://dx.doi.org/10.1016/j.optlastec.2008.08.007 OLTCAS 0030-3992 Google Scholar
J. Diaci,
“Laser profilometry for the characterization of craters produced in hard dental tissues by Er:YAG and Er,Cr:YSGG lasers,”
J. Laser Health Acad., 2008
(2), 1
–9
(2008). Google Scholar
D. BračunM. JezeršekJ. Diaci,
“Triangulation model taking into account light sheet curvature,”
Meas. Sci. Technol., 17
(8), 2191
–2196
(2006). http://dx.doi.org/10.1088/0957-0233/17/8/019 MSTCEP 0957-0233 Google Scholar
P. GregorčičM. JezeršekJ. Možina,
“Optodynamic energy-conversion efficiency during an Er:YAG-laser-pulse delivery into a liquid through different fiber-tip geometries,”
J. Biomed. Opt., 17
(7), 075006
(2012). http://dx.doi.org/10.1117/1.JBO.17.7.075006 JBOPFO 1083-3668 Google Scholar
J. ArendsJ. L. RubenD. Inaba,
“Major topics in quantitative microradiography of enamel, and dentin: R parameter, mineral distribution visualization, and hyper-remineralization,”
Adv. Dent. Res., 11
(4), 403
–414
(1997). http://dx.doi.org/10.1177/08959374970110040501 0895-9374 Google Scholar
K. NemešM. LukačJ. Možina,
“Variable square pulse vs conventional PFN pumping of Er:YAG laser,”
Opt. Laser Technol., 44
(3), 664
–668
(2012). http://dx.doi.org/10.1016/j.optlastec.2011.09.020 OLTCAS 0030-3992 Google Scholar
A. Mehlet al.,
“3D volume-ablated rate, and thermal side effects with the Er:YAG, and Nd:YAG,”
Dent. Mater., 13
(4), 246
–251
(1997). http://dx.doi.org/10.1016/S0109-5641(97)80036-X DEMAEP 0109-5641 Google Scholar
M. Ithet al.,
“Dynamics of laser-induced channel formation in water and influence of pulse duration on the ablation of biotissue under water with pulsed erbium-laser radiation,”
Appl. Phys. B, 59
(6), 621
–629
(1994). http://dx.doi.org/10.1007/BF01081183 APBOEM 0946-2171 Google Scholar
H. W. Kanget al.,
“Hard tissue ablation with a spray-assisted mid-IR laser,”
Phys. Med. Biol., 52
(24), 7243
–7259
(2007). http://dx.doi.org/10.1117/12.273593 PSISDG 0277-786X Google Scholar
M. Miret al.,
“Visualizing the procedures in the influence of water on the ablation of dental hard tissue with erbium:yttrium-aluminium-garnet, and erbium, chromium:yttrium-scandium-gallium-garnet laser pulses,”
Lasers Med. Sci., 24
(3), 365
–374
(2009). http://dx.doi.org/10.1007/s10103-008-0571-1 LMSCEZ 1435-604X Google Scholar
M. Miret al.,
“Influence of water-layer thickness on Er:YAG laser ablation of enamel of bovine anterior teeth,”
Lasers Med. Sci., 23
(4), 451
–457
(2008). http://dx.doi.org/10.1007/s10103-007-0508-0 LMSCEZ 1435-604X Google Scholar
G. H. Didbin,
“The water in human dental enamel, and its diffusional exchange measured by clearance of tritiated water from enamel slabs of varying thickness,”
Caries Res., 27
(2), 81
–86
(1993). http://dx.doi.org/10.1159/000261522 CAREBK 0008-6568 Google Scholar
L.C. Courrolet al.,
“Spectroscopic study of ejected dental tissue after Er:YAG laser ablation,”
J. Lumin., 102–103 96
–100
(2003). http://dx.doi.org/10.1016/S0022-2313(02)00473-8 JLUMA8 0022-2313 Google Scholar
B. Majaronet al.,
“Heat diffusion, and debris screening in Er:YAG laser ablation of hard biological tissues,”
Appl. Phys. B, 66
(4), 479
–487
(1998). http://dx.doi.org/10.1007/s003400050423 APBOEM 0946-2171 Google Scholar
K. L. Vodopyanov,
“Bleaching of water by intense light at the maximum of the absorption band,”
Sov. Phys. JETP, 70
(1), 114
–121
(1990). SPHJAR 0038-5646 Google Scholar
J. P. CummingsJ. T. Walsh,
“Erbium laser ablation: the effect of dynamic optical properties,”
Appl. Phys. Lett., 62
(16), 1988
–1990
(1993). http://dx.doi.org/10.1063/1.109512 APPLAB 0003-6951 Google Scholar
D. Friedet al.,
“Infrared radiometry of dental enamel during Er:YAG, and Er:YSGG laser irradiation,”
J. Biomed. Opt., 1
(4), 455
–465
(1996). http://dx.doi.org/10.1117/12.250668 JBOPFO 1083-3668 Google Scholar
T. Perhavecet al.,
“Heat deposition of erbium lasers in hard dental tissues,”
J. Oral Laser Appl., 9
(4), 205
–212
(2009). 1473-7809 Google Scholar
R. K. Shoriet al.,
“Quantification, and modeling of the dynamic changes in the absorption coefficient of water at ,”
IEEE J. Sel. Top. Quantum Electron., 7
(6), 959
–970
(2001). http://dx.doi.org/10.1109/2944.983300 IJSQEN 1077-260X Google Scholar
K. StockR. HibstU. Keller,
“Comparison of Er:YAG and Er:YSGG laser ablation of dental hard tissues,”
Proc. SPIE, 3192 88
–95
(2000). http://dx.doi.org/10.1117/12.297864 PSISDG 0277-786X Google Scholar
A. V. Belikovet al.,
“Comparative study of the 3 micron laser action on different hard tissue samples using free running pulsed Er-doped YAG, YSGG, YAP and YLF lasers,”
Proc. SPIE, 2080 60
–67
(1993). http://dx.doi.org/10.1117/12.166167 PSISDG 0277-786X Google Scholar
|