|
|
1.IntroductionThe topicality of the study of protein molecules is related to their decisive role in biochemical processes in living matter. The interpretation of such processes necessitates comprehensive characterization of the functional activity of enzymes, which can be considered as macromolecular machines with specific functions. The enzymatic activity significantly depends on the molecular structure. Even minor structural modifications of an enzyme may lead to substantial changes of conformational dynamics and, hence, functional activity. Several methods can be used to modify protein structure. The structure of the surface amino acid residues can easily be changed using variations in the native (aqueous) environment of a protein molecule. In particular, the state of surface amino groups of -chymotrypsin (CT) is modified when the native environment (water) is changed by organic solvents and the functional activity is transformed from the hydrolysis of peptide bonds into transesterification with a significant decrease in the catalytic activity.1–3 The presence of crown-ether molecules in organic solvents leads to an increase in the activity by several orders of magnitude due to the interaction with surface amino groups.4 Thermal denaturation evidently leads to modification of protein structure. The changes of FTIR and Raman spectra of CT upon thermal denaturation have been studied in Ref. 5. The spectral changes show that the denaturation causes changes in the secondary structure, conformation of tyrosine residues, and conformation of disulfide bridges. Several chemical agents specifically interact with the structural elements of a protein globule. In particular, tris(2-carboxyethyl)phosphine (TCEP) and dithiothreitol (DTT) provide the cleavage of disulfide bonds and the corresponding spectral changes have been studied in Refs. 67.–8. Enzyme functioning can be terminated using specific agents (inhibitors) that interact with the active site. In particular, phenylmethanesulfonylfluoride (PMSF) may serve as the inhibitor for CT.9,10 FTIR and Raman spectroscopic techniques are known to be efficient tools in the study of function-related structural changes of protein molecules. Conformation-sensitive amide I () and amide III () bands can be used to characterize the secondary structure of a protein molecule. The bands assigned to the disulfide bridges are detected in the spectral interval of 500 to . Relative intensities of the tyrosine doublet (830 and ) are sensitive to H-bonding. These and several additional spectral features from the fingerprint range are normally used in the study of protein molecules. However, there has been considerable recent interest in the spectral measurements in the low-frequency (50 to ) range. (One of the reasons is the significant progress in the terahertz spectroscopy, which can be considered in several applications as a modification of the FTIR spectroscopy.) Subglobular and cooperative oscillations of macromolecules must be manifested in this range11–13 and, hence, conformational changes of protein molecules can additionally be characterized using the spectroscopic techniques. Several technical problems of the spectroscopy in the low-frequency range and the problems related to the analysis and interpretation of spectra containing relatively broad overlapped bands impede the application of the potentially informative spectral interval in the analysis of protein conformational changes. Only in a few works are spectral bands assigned to specific vibrational modes of protein molecules (see, for example, Refs. 14 and 15). Note that contradictory interpretations are sometimes proposed in the literature. Thus, the analysis of vibrational spectra of protein molecules in the low-frequency range and application of the corresponding results in the study of the function-related structural changes are topical problems. In this work, we measure the low-frequency (100 to ) FTIR and Raman spectra of native proteins [CT and bovine serum albumin (BSA)] and samples in which protein molecules are modified due to thermal denaturation, cleavage of disulfide bridges, or inhibition. The similarities and differences of spectral changes are discussed and attempts at interpretation of the spectral data are made. 2.ExperimentalIn the experiments, we use chymotrypsin from Samson-Med (CAS #9004-07-3) and BSA from MP Biomedicals (CAS #9048-46-8). The molecular masses of the native proteins are and , and the numbers of disulfide bridges are and .16 The dominant element in the secondary structure of BSA is -helix, whereas CT is almost free of -helices and the contents of -sheets and random coils in it are almost equal.16 For cleavage of disulfide bridges, we employ DTT from Sigma (CAS #3483-12-3) and TCEP from Thermo Scientific (CAS #51805-45-9). CT is inhibited using PMSF from Helicon (CAS #329-98-6). Thermally denatured BSA and CT are obtained using 90-min heating of aqueous solutions of proteins () at 80°С and subsequent lyophilization. For inhibition, CT aqueous solution at a concentration of is mixed with PMSF solution in acetonitrile (acetonitrile prevents hydrolysis of PMSF prior to mixing). The protein-to-PMSF molar ratio in the solution is . Then, the mixture is lyophilized. Proteins are denatured using TCEP and DTT with the aid of the procedures of Refs. 6 and 8, respectively. The protein-to-TCEP and protein-to-DTT molar ratios are and , respectively. For Raman measurements, lyophilized powders are used. For FTIR measurements, the lyophilized samples are pressed into tablets. For FTIR measurements, we use a Thermo Scientific Nicolet-6700 spectrometer. Each spectrum results from averaging over 300 scans. The measurement interval is 100 to , and the resolution is . Relatively high absorption coefficients of the samples under study necessitate a decrease in the thickness of tablets to several tens of microns. Such thin tablets are mechanically unstable, and we mix lyophilized proteins with paraffin at a protein-to-paraffin ratio of . Thus, the samples for FTIR measurements represent protein-paraffin tablets with a mass of 35 to 40 mg, a diameter of 13 mm, and a thickness of that are pressed at a pressure of . The FTIR spectrum of paraffin in the spectral interval under study is free of developed bands, and the paraffin signal represents a relatively low background in the spectra of the protein-paraffin tablets. For Raman measurements, we use a Thermo Scientific DXR Raman confocal microscope. The excitation wavelength is 532 nm, mean power at the sample is no greater than 10 mW, and the spectral resolution is (the dispersion is per pixel). In the experiments, we use lyophilized powders. To analyze the FTIR data, we follow the approach of Ref. 14 and fit the spectra using Lorentzian curves to determine the peak positions. Such a procedure is needed since the FTIR spectra of the samples under study contain relatively broad overlapped spectral bands. We use the initial set of the Lorentzian components of Ref. 14, where the FTIR spectra of several proteins (trypsin, lysozyme, and BSA) have been fitted. For several samples, a few bands were added to appropriately fit the measured spectra. Below, we use the positions of the fitting Lorentzian components in the analysis of the FTIR spectra. The measured Raman spectra are -converted.17 Then, the background is subtracted,18 and the spectra are smoothed using 20-points Savitzky-Golay filter. To determine the positions of the spectral bands in both Raman and FTIR spectra, we additionally use the second derivatives of the spectral curves. 3.ResultsFigure 1(a) presents the FTIR spectra of BSA in the spectral interval of 100 to . For comparison, the spectra are arbitrarily shifted along the vertical axis. It is seen that the samples exhibit broad asymmetric spectral bands in the interval of 100 to that cannot be fitted using a single Lorentzian component. At least two Lorentzian components in this interval are needed for the approximation. In particular, the peak positions for the native BSA are 118 and . The position of the first peak remains unchanged for the remaining samples, whereas the position of the second component ranges from 159 to . Note also additional variations in the widths and relative amplitudes. The bands in this interval can be assigned to the NH out-of-plane bending, CN torsion, or out-of-plane bending.14 Fig. 1FTIR spectra of [(a) to (c)] bovine serum albumin (BSA) and [(d) to (f)] chymotrypsin (CT) samples: (1) tris(2-carboxyethyl)phosphine (TCEP)-protein complexes, (2) thermally denatured proteins, (3) dithiothreitol (DTT)-protein complexes, (4) native proteins, and (5) inhibited CT. 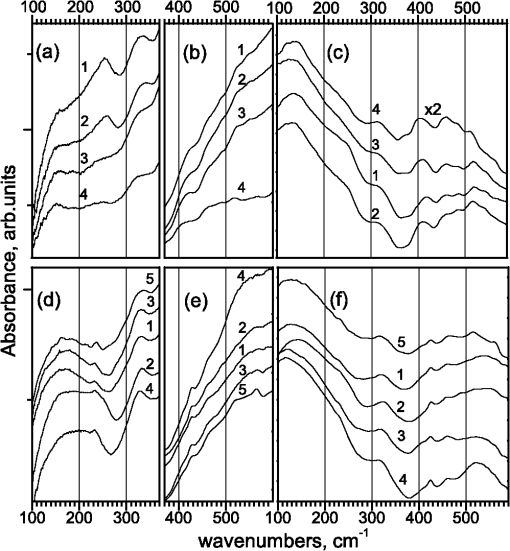 Similar results are obtained for CT [Fig. 1(d)]. The positions of the fitting components for the native protein are 115 and . Almost the same positions are obtained for the sample with TCEP and denatured protein although the relative amplitudes are different. For the sample with DTT, the fitting components are shifted to 109 and , respectively. For the inhibited protein, the peak positions are 121 and . For both proteins, the widths of the fitting Lorentzian components are . Significant changes are observed in the BSA spectra in the interval of 200 to . The spectrum of the native protein exhibits a very weak band. The interaction with DTT leads to an increase in the band at . The band further increases in the thermally denatured sample and the sample with TCEP. The band at 250 to has been assigned to the bending in Ref. 15. Each CT sample exhibits a band at . In comparison with the spectrum of the native CT [curve 4 in Fig. 1(d)], all of the remaining samples exhibit an increase in the intensity at (similar to the BSA samples). The band at has been assigned to the methyl torsion in Ref. 2. A weak band at in the spectrum of the inhibited CT can be assigned to the deformation or torsion.2 The band at in the spectra of all samples can be assigned to the CNC deformation, in-plane bending, and CCN deformation.1 Figures 1(b) and 1(e) show the FTIR spectra in the interval of 370 to . The presence of significant almost linear backgrounds impedes the analysis of spectral changes. Therefore, we additionally process the experimental data using double differentiation and double integration of the curves with subtracting mean levels prior to integration. Such a procedure makes it possible to delete constant and linear background components. The processed spectra are presented in Figs. 1(c) and 1(f). For comparison, curve 4 in Fig. 1(c) (native BSA) is scaled up by a factor of 2. An asymmetric spectral feature is observed in the BSA spectra in the interval of 380 to . The modifications of the protein molecule lead to the shift of the spectral maximum from 403 to if the samples are ordered in the following way: native protein, sample with DTT, thermally denatured sample, and sample with TCEP. The bands in this interval are assigned to side-chain vibrations.2 A developed peak at and a shoulder at are observed in the CT spectra. Based on this result, we may assume that the same doublet is observed in BSA spectra and the visible shift of the spectral maximum is due to a decrease in the relative intensity of the low-frequency component. Such a decrease is also observed in Fig. 1(f) for almost the same order of the samples. The spectrum of the inhibited CT is most similar to the spectrum of the sample with TCEP. The redistribution of the relative intensities is also observed for the bands at 460 and in the BSA spectra. These bands are assigned to the bending and side-chain vibrations, respectively.2 Both bands are clearly visible only in the spectrum of the thermally denatured CT. The remaining CT samples exhibit only the band at . The BSA spectra exhibit the band at . For the above order of the samples, we observe an increase in the relative intensity of the band at . The spectrum of the native BSA exhibits the band at , which is almost undetectable in the spectra of the remaining samples. In the spectral interval of 500 to , the spectrum of the native CT substantially differs from the spectra of the remaining samples. The original spectra in Fig. 1(e) show an increase in the relative intensity of the band at in the modified samples, and the most developed band corresponds to the inhibited CT. To additionally characterize protein changes related to the interaction with reagents, we employ Raman spectroscopy. Figure 2 shows the processed Raman spectra that are arbitrarily shifted along the vertical axis. Fig. 2Raman spectra of [(a) and (b)] BSA and [(c) and (d)] CT samples: (1) TCEP-protein complexes, (2) thermally denatured proteins, (3) DTT-protein complexes, (4) native proteins, and (5) inhibited CT. 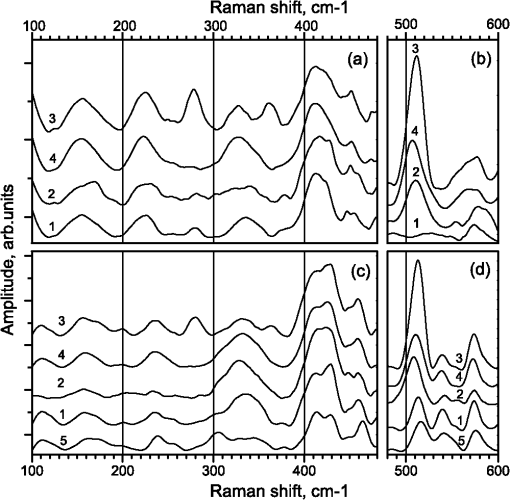 Figures 2(a) and 2(b) present the spectra of native and modified BSA. An asymmetric spectral feature at that presumably consists of several spectral components is observed in all spectra. The interactions with reagents cause redistribution of intensities and, hence, variations in the band shape. Note an increase in the intensity at , which is most developed in the spectrum of the thermally denatured sample. This sample also exhibits a small band at , which is not observed in the spectra of the remaining samples. The Raman bands at frequencies of in the CT spectra are also sensitive to interactions. In particular, the band of native protein at vanishes in the spectrum of the denatured sample and the intensity of the broad band at decreases. In the remaining modified samples, we observe an increase in the intensity at . The thermal denaturation of BSA also leads to noticeable changes of the broad band at : the intensity of the low-frequency (high-frequency) component decreases (increases). This band is slightly shifted in the TCEP-protein sample and remains almost unchanged in the DTT-protein sample. The broad band at in the spectrum of the native CT is transformed into a doublet in the spectra of the inhibited protein and TCEP-protein complex. As in the spectra of albumin, the thermal denaturation causes a significant decrease in the intensity of the band and the interaction with DTT does not cause significant changes. The thermal denaturation causes significant changes of the BSA band at . The interaction with TCEP leads to the shift of this band to , and the interaction with DTT results in the changes of the band shape. The broad band at in the CT spectrum is similar to the corresponding band in the BSA spectrum, but a developed shoulder appears at in the former spectrum. The spectral component at becomes dominant in the spectrum of inhibited CT. The redistribution of the relative intensities of spectral components in the frequency interval of 300 to is observed in the spectra of the denatured sample and the sample with TCEP. In the spectra of both proteins, the interaction with DTT predominantly causes a decrease in the total intensity of the band at 330 to . Note that the bands at 280 and 360 to appear in the spectra of modified proteins. The most intense bands at 280 and are observed in the spectra of the DTT-protein samples. The intensity of the band at is also significant in the spectra of the TCEP-BSA and denatured BSA samples. The spectra of the modified proteins (except for the DTT-protein samples) exhibit the spectral bands shifted to . The bands at 280 and 360 to cannot be assigned to the reagents, since the characteristic bands of reagents are not observed in the spectra at the experimental concentrations. The corresponding measurements in the fingerprint range have also proven the absence of the characteristic bands of reagents. A doublet at 410 and is observed in the spectra of all samples. As for several bands above, the interaction with DTT does not lead to significant spectral changes. However, the spectra of the remaining modified samples exhibit a decrease in the low-frequency component of the doublet and an increase in the high-frequency component. A single peak at in the spectra of the native BSA and DTT-BSA sample is transformed into a doublet in the spectra of the remaining modified samples with the second component at . Almost the same doublet is observed in the spectra of the CT samples but the high-frequency component dominates. Only in the spectrum of the native CT is the doublet not developed and the high-frequency component is observed at . In the modified samples, the intensity of the low-frequency component increases. In general, the thermal denaturation causes the most significant changes in the Raman spectra in the interval of 100 to . The bands assigned to disulfide bridges are observed in the spectral interval of 500 to [Figs. 2(b) and 2(d)]. It is commonly accepted that the bands at 510, 525, and are assigned to the gauche-gauche-gauche, gauche-gauche-trans, and trans-gauche-trans conformations of S-S bridges.19 The spectrum of the native BSA exhibits a single band at , which indicates the dominant gauche-gauche-gauche conformation of the disulfide bridges. The thermal denaturation causes a minor blue shift of the band and, hence, minor modification of the conformation of bridges. The effect of DTT involves the cleavage of the disulfide bonds in the protein molecule and the formation of such bridges in the oxidized DTT (trans-4,5-dihydroxy-1,2-dithiane). Thus, we observe an increase in the intensity of the blue-shifted band, which must be assigned to the oxidized DTT rather than the protein. TCEP also provides the cleavage of disulfide bonds, but, in this case, new bridges are not formed and we observe a dramatic decrease in the intensity in the spectral interval of 500 to , which indicates almost complete absence of disulfide bridges in the TCEP-BSA sample. The Raman spectra of CT samples show that the denaturation weakly affects the conformations of the disulfide bridges. The inhibition and the interaction with TCEP lead to the shift of the most intense band from 510 to and an increase in the relative intensity of the band at . The intensity of the band at in the Raman spectrum of the DTT-CT sample increases due to the S-S bridges in the oxidized DTT, but the intensity of the band at remains almost unchanged. Thus, the results for DTT-CT and TCEP-CT samples indicate that CT is more stable than BSA against the agents that provide the cleavage of disulfide bonds. 4.DiscussionThe low-frequency FTIR and Raman spectra of BSA and CT are generally similar. However, developed spectral differences can be due to significantly different structures of the two proteins. The analysis of the FTIR and Raman spectra shows that the most significant structural changes of the proteins result from thermal denaturation. The changes of several bands in the spectra of modified proteins are similar. In particular, the FTIR data for CT show that the amplitude of the band at 255 to increases if the samples are ordered in the following way: native protein, sample with DTT, sample with TCEP, and thermally denatured protein. The intensity of this band in the BSA spectra also increases for almost the same order: native protein, sample with DTT, thermally denatured protein, and sample with TCEP. Note that the bands in the spectra of the last two samples are almost identical so that the orders for the two proteins are virtually identical. When the spectra are ordered in the same way, the intensity of the band at decreases for both proteins. For the above order of the CT samples, we observe an increase in the intensity of the Raman band at relative to the intensity of the band at . The bands at 225 and in the Raman spectra of BSA exhibit the same transformations. The bands at 410 and are observed in the Raman spectra of both proteins. For BSA (CT) samples, the intensity of the low-frequency (high-frequency) component is higher. The same relationship of the intensities is valid for the bands at 450 and . For the above order of the samples, the intensity of the band at 450 increases in the Raman spectra of CT (BSA). A relatively small PMSF (inhibitor) molecule () interacts with the CT active site and binding pocket. Such interaction can be classified as local with respect to the protein molecule as a whole and similarity of the spectra of native and inhibited CT can be expected. However, the spectra of inhibited CT noticeably differ from the spectra of the native protein, which indicates that the ligand binding induces significant conformational changes of the protein molecule in agreement with the general concept of the structurefunction relationship. The spectral changes resulting from CT inhibition are similar to the spectral changes due to CT inhibition with an alternative ligand (anthranilic acid).20 In both cases, the redistribution of relative intensities at 510 and can be due to transformation from the gauche-gauche-gauche to trans-gauche-trans conformation. Such a similarity is an expected result, since the conformations of the S-S bridges, which are not in direct contact with the CT active site, must not be sensitive to a specific ligand. Raman spectra of DTT-protein and TCEP-protein samples significantly differ from Raman spectra of native proteins. Raman data show that (1) TCEP provides almost complete cleavage of disulfide bridges in BSA and (2) the cleavage of S-S bridges in proteins due to the effect of DTT is accompanied by the formation of the bridges in the oxidized DTT. Taking into account the absence of significant changes in FTIR spectra of the same samples, we may conclude that the bands of disulfide bridges are not observed in FTIR spectra. In accordance with the published experimental data and model calculations, amide VI and amide VII bands must be observed in spectral intervals of 500 to 610 and 160 to , respectively.21–25 Both Raman and FTIR spectra of BSA differ from the corresponding spectra of CT in the above intervals (recall that the secondary structures of the two proteins are significantly different). Interaction with chemical agents and thermal denaturation also lead to spectral changes in the above intervals. However, only tentative assignments can be found in the literature and the results on the relation of the corresponding band shapes and contents of secondary-structure elements are missing. Thus, the existing data are insufficient for analysis in terms of amide bands. 5.ConclusionsBased on the differences between the low-frequency (100 to ) vibrational spectra of proteins with significantly different structures, we assume that the spectral interval can be used to characterize protein conformations. The experimentally observed correlated variations in the intensities of several low-frequency bands in the series natural protein, DTT-protein sample, TCEP-protein sample, and denatured protein may indicate increasing conformational changes in such a series. The strongest spectral changes are caused by thermal denaturation of the protein samples. However, comparable changes result from the cleavage of disulfide bonds. In spite of the local character of the PMSF interaction with CT, the ligand binding induces significant spectral changes. Presumably, the corresponding conformational changes of the protein molecule can be compared to the changes induced by thermal denaturation. AcknowledgmentsThis work was supported by the Russian Foundation for Basic Research (Grant No. 13-02-01177) and Lomonosov Moscow State University Program of Development. ReferencesN. N. BrandtA. Y. ChikishevI. K. Sakodynskaya,
“Raman spectroscopy of tris-(hydroxymethyl)aminomethane as a model system for the studies of α-chymotrypsin activation by crown ether in organic solvents,”
J. Mol. Struct., 648
(3), 177
–182
(2003). http://dx.doi.org/10.1016/S0022-2860(03)00020-6 JMOSB4 0022-2860 Google Scholar
A. A. Mankovaet al.,
“Terahertz time-domain and FTIR spectroscopy of tris-crown interaction,”
Chem. Phys. Lett., 554 201
–207
(2012). http://dx.doi.org/10.1016/j.cplett.2012.10.039 CHPLBC 0009-2614 Google Scholar
A. A. Mankovaet al.,
“Terahertz time-domain and FTIR spectroscopic study of interaction of a-chymotrypsin and protonated tris with 18-crown-6,”
Chem. Phys. Lett., 560 55
–59
(2013). http://dx.doi.org/10.1016/j.cplett.2012.12.050 CHPLBC 0009-2614 Google Scholar
J. Brooset al.,
“Large activation of serine proteases by pretreatment with crown ethers,”
J. Chem. Soc., Chem. Commun., 2 255
–256
(1995). http://dx.doi.org/10.1039/c39950000255 CCJDAO 0577-6171 Google Scholar
N. N. Brandtet al.,
“FTIR and THz spectroscopy of chymotrypsin upon interaction with crown ether, denaturation, and inhibition,”
Biomed. Spectrosc. Imaging, 3 219
–224
(2014). BSIIAX 2212-8794 Google Scholar
C. DavidS. FoleyM. Enescu,
“Protein S-S bridge reduction: a Raman and computational study of lysozyme interaction with TCEP,”
Phys. Chem. Chem. Phys., 11 2532
–2542
(2009). http://dx.doi.org/10.1039/b815492a PPCPFQ 1463-9076 Google Scholar
E. B. Getzet al.,
“A comparison between the sulfhydryl reductants tris(2-carboxyethyl)phosphine and dithiothreitol for use in protein biochemistry,”
Anal. Biochem., 273 73
–80
(1999). http://dx.doi.org/10.1006/abio.1999.4203 ANBCA2 0003-2697 Google Scholar
C. Davidet al.,
“Reductive unfolding of serum albumins uncovered by Raman spectroscopy,”
Biopolymers, 89
(7), 623
–634
(2008). http://dx.doi.org/10.1002/(ISSN)1097-0282 BIPMAA 0006-3525 Google Scholar
G. T. James,
“Inactivation of the protease inhibitor phenylmethylsulfonyl fluoride in buffers,”
Anal. Biochem., 86 574
–579
(1978). http://dx.doi.org/10.1016/0003-2697(78)90784-4 ANBCA2 0003-2697 Google Scholar
Y. I. KhurginE. Y. Maksareva,
“Study of the solid-state enzyme reactions. 3. Irreversable inactivation of -chymotrypsin by benzylsulfonyl fluoride,”
Boioorganicheskaya khimiya, 17
(1), 76
–80
(1991). Google Scholar
A. I. Kitaigorodsky, Molecular Crystals, Nauka, Moscow
(1971). Google Scholar
M. C. BeardG. M. TurnerC. A. Schmuttenmaer,
“Measuring intramolecular charge transfer via coherent generation of THz radiation,”
J. Chem. Phys. A, 106 878
(2002). http://dx.doi.org/10.1021/jp013603l JPAHER 1155-4312 Google Scholar
R. J. FalconerA. G. Markelz,
“Terahertz spectroscopic analysis of peptides and proteins,”
J. Infrared Milli Terahertz Waves, 33 973
(2012). http://dx.doi.org/10.1007/s10762-012-9915-9 1866-6892 Google Scholar
Ch. U. Stehleet al.,
“Far-infrared spectroscopy on free-standing protein films under defined temperature and hydration control,”
J. Chem. Phys., 136 075102
(2012). http://dx.doi.org/10.1063/1.3686886 JCPSA6 0021-9606 Google Scholar
J. Twardowski,
“Far infrared spectra of acid phosphatase from rat liver. Spectra of the native and the heat- and acid-denatured isoenzymes,”
Acta biochimica polonica, 27
(1), 1
–7
(1980). ABPLAF 0001-527X Google Scholar
O. F. Nielsen,
“Low frequency spectroscopic studies of interactions in liquids,”
Annu. Rep. Prog. Chem. C Phys. Chem., 90 3
–44
(1993). http://dx.doi.org/10.1039/PC9939000003 Google Scholar
Ch. A. LieberA. Mahadevan-Jansen,
“Automated method for subtraction of fluorescence from biological Raman spectra,”
Appl. Spectrosc., 57
(11), 1363
–1367
(2003). http://dx.doi.org/10.1366/000370203322554518 APSPA4 0003-7028 Google Scholar
F. S. Parker, Applications of Infrared, Raman, and Resonance Raman Spectroscopy in Biochemistry, Plenum Press, New York
(1983). Google Scholar
N. N. Brandtet al.,
“CARS and Raman spectroscopy of function-related conformational changes of chymotrypsin,”
J. Raman Spectrosc., 31 731
–737
(2000). http://dx.doi.org/10.1002/(ISSN)1097-4555 JRSPAF 0377-0486 Google Scholar
H. Susi,
“The strength of hydrogen bonding: infrared spectroscopy,”
Methods Enzymol., 26 381
–391
(1972). http://dx.doi.org/10.1016/S0076-6879(72)26019-0 MENZAU 0076-6879 Google Scholar
Y. El Khouryet al.,
“On the specificity of the amide VI band for the secondary structure of proteins,”
Vibr. Spectrosc., 55
(2), 258
–266
(2011). http://dx.doi.org/10.1016/j.vibspec.2010.12.001 VISPEK 0924-2031 Google Scholar
A. ElliottE. J. Ambrose,
“Structure of synthetic polypeptides,”
Nature, 165 921
–922
(1950). http://dx.doi.org/10.1038/165921a0 NATUAS 0028-0836 Google Scholar
S. KrimmJ. Bandekar,
“Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins,”
Adv. Protein Chem., 38 181
–364
(1986). http://dx.doi.org/10.1016/S0065-3233(08)60528-8 APCHA2 0065-3233 Google Scholar
G. G. SuchkovaL. I. Maklakov,
“Amide bands in the IR spectra of urethanes,”
Vibr. Spectrosc., 51 333
–339
(2009). http://dx.doi.org/10.1016/j.vibspec.2009.09.002 VISPEK 0924-2031 Google Scholar
BiographyNikolay N. Brandt graduated and received his PhD degree from the Faculty of Physics, Moscow State University in 1998 and 2001, respectively. He is a participant of International Research Projects in Tokyo and Berlin. He is an associate professor at the Faculty of Physics, Moscow State University. His areas of expertise are Raman, IR, and fluorescence spectroscopy of biological macromolecules aimed at the analysis of the relationship of conformational dynamics and functional activity. Andrey Yu Chikishev graduated and received his PhD and DrSci degrees from the Faculty of Physics, Moscow State University, in 1982, 1986, and 2002, respectively. He is a leader and participant of International Research Projects on laser spectroscopy of biomolecules and organic molecules in the Netherlands, Germany, Japan, and Italy. He is a head of the Laboratory of Laser Diagnostics of Biomolecules and Methods of Photonics in the study of objects of cultural heritage, International Laser Center, Moscow State University. Anna A. Mankova graduated from the Faculty of Physics, Moscow State University in 2012. Since 2012 she has been a PhD student at the Faculty of Physics, Moscow State University. Her area of interest is low-frequency spectroscopy of biological molecules. Inna K. Sakodynskaya graduated and received his PhD degree from the Chemistry Department at Moscow State University in 1981 and 1985, respectively. He is a participant of International Research Projects in the Netherlands, United States, and Italy. He is a leading researcher in the Chemistry Department, Moscow State University. His areas of expertise are kinetics and catalysis, chemical enzymology. |

