|
|
1.IntroductionFluorescence cystoscopy (FC) efficiently enhances the detection and improves the therapeutic outcome of early bladder cancer.1–5 Nowadays, it is performed using blue–violet (typically between 380 and 435 nm) light to induce the red (610 to 720 nm) fluorescence of photoactivable porphyrins (PaPs), including Protoporphyrin IX (PpIX), produced endogenously in the cancerous cells of the bladder wall (for the purpose of this paper, “PPIX” will be used throughout as a proxy for all PaPs, of which PPIX is the most widely studied). This follows instillation of 50 ml of a precursor solution,5,6 usually hexylaminolevulinate (Hexvix® as the commercial name), during 1 to 2 h.7 The resulting fluorescence images produced by commercially available instruments often have a blue–green color for healthy tissues, while the early cancerous lesions appear in red. During an FC, about 150 ml of water is needed to inflate the bladder after its drainage to remove the Hexvix® solution. Concomitantly, the kidneys produce urine with an average flow of 1 to 2 ml/min. Hence, the water instilled during the FC rapidly contains a non-negligible amount of urine. If the urine is fluorescent, making the bladder washout fluid (BWF) fluorescent as a consequence, the FC images are frequently degraded in a manner detrimental to the FC and to the clarity of the observation field. The degradation of FC images can result in various effects: loss of contrast, whereby lesions are more difficult to spot amidst a generally fluorescent environment; screening of the bladder wall, whereby photons that would normally excite the fluorescence of the bladder wall are absorbed by the medium filling the bladder and do not reach their target. In both cases, the otherwise optimal contrast between normal tissue and early lesions is lost, or at least severely reduced, thus impairing the optimal detection of the latter. Figure 1 shows clinical images taken during cystoscopies in conditions of good visibility and under suboptimal conditions (courtesy of Prof. P. Jichlinski, CHUV University Hospital, Lausanne, Switzerland). Fig. 1(a) Clinical images taken during cystoscopies of conditions of good visibility; (b) greenish fluorescence of urine exiting the ureter into the bladder; and (c) background fluorescence of bladder washout fluid. 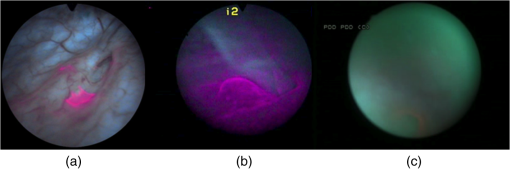 It is clear from Fig. 1 that the urine produced during the examination can be fluorescent, with spectral properties that make it interfere with the cystoscopy. It is thus of high interest to minimize this perturbation, for instance, by controlling the dietary intake of molecules whose metabolites will make urine fluorescent. Several fluorophores may cause urine’s background fluorescence, but their exact origin and relative contribution are not fully understood. It is, however, likely that the main peak of fluorescence be due to 4-pyridoxic acid (excitation around 317 nm and emission around 423 nm)8–11 and that some other metabolites contribute to a pair of weaker, secondary peaks (excitation wavelength: 370 nm/emission wavelength: 525 nm; excitation wavelength: 450 nm/emission wavelength: 525 nm), which are more diet dependent than the main peak. Unfortunately, it is unclear which foods, drinks, drugs, or over-the-counter (OTC) tablets may contribute to the background fluorescence. It is, however, clear that this background can be detected during FC and that it jeopardizes the benefits otherwise offered by the method in many cases. One way to minimize this background is to frequently drain the BWF during FC (or to use a system that allows continuous flow). This procedure is not complicated per se, but it makes the FC longer and more tedious for the operator. We expect that several types of dietary intakes impact urine fluorescence and propose to start this exploration with OTC vitamin supplements because they are widely used in the general population. They are also commonly given to hospitalized patients subject to FC.12 Thus, the main purpose of this study is to explore the impact of a commercial cocktail of OTC vitamin supplements on the urine fluorescence of healthy volunteers. To this end, we measured excitation–emission matrices (EEMs) of urine samples and the kinetics of modifications of urine fluorescence before, during, and after vitamin intake. Our measurements give an initial indication of how to minimize the perturbations resulting from urine fluorescence during FC. They also help to issue recommendations to the clinicians as to which OTC vitamin supplements should be avoided in the days leading to an FC. Finally, they suggest straightforward ways to optimize the spectral design of imaging systems for FC to minimize perturbations induced by the ingestion of OTC vitamin supplements cocktails containing vitamins of the B group. 2.Materials and Methods2.1.Volunteers and Urine SamplesThe urine samples were obtained from nine healthy volunteers [five males, four females, mean age: 38.5 years old (24 to 70 years old)], who gave their informed consent to participate in the study. On any sampling day, volunteers were asked to provide one sample of urine in the morning and one in the afternoon. To establish the baseline of the measurements, volunteers were initially asked to provide samples of pure urine during 7 days. Then they were supplied with OTC vitamin supplements (Berocca, Bayer AG, Zurich, Switzerland) and asked to take one tablet/day during 1 week, in the morning, as per recommended doses. Each tablet of the OTC vitamin supplement is composed of: vitamin B1 (15 mg), vitamin B2 (15 mg), vitamin B6 (10 mg), vitamin B12 (), vitamin C (500 mg), biotin (), folic acid (), nicotinamid (50 mg), pantothenic acid (23 mg), calcium (100 mg), magnesium (100 mg), and zinc (10 mg). Urine samples were collected every other working day during that week (Monday, Wednesday, and Friday) and for 4 days after volunteers stopped taking vitamins, to monitor the return of urine fluorescence to baseline values. This study was performed in compliance with the local regulations and was approved by the Local Ethics Committee of the HUG Geneva University Hospital. 2.2.Sample PreparationAll urine samples were stored at 4°C in the dark between collection and the spectral measurements. The typical time interval between collection and measurement was 2 h. Approximately 1 ml of noncentrifuged urine was diluted with purified water (Aqua, B. Braun, Germany) by a factor 25 (1 ml of urine sample and 25 ml of Aqua). In this study, because we collected samples from healthy volunteers (and not from patients undergoing a cystoscopy), we did not per se study BWF but rather diluted urine. It should be noted that the samples of diluted urine were similar to BWF insofar as they are urine diluted with water.10 This approach presents, in addition, the advantage of avoiding the bias generated by the inner filter effects frequently observed during fluorescence spectroscopy.10 2.3.SpectroscopyApproximately 3.5 ml of the diluted urine samples were placed in a quartz cuvette (Hellma OS, Germany). The fluorescence was analyzed with a spectrofluorometer (FluoroLog 3-22, Horiba, Jobin Yvon, France) and the results were presented in the form of an EEM. The excitation wavelengths ranged between 300 and 500 nm (5 nm increments; excitation slit: 2 nm) and emission wavelengths ranged between 300 and 700 nm (5 nm increments; emission slit: 2 nm). Fluorescence was collected with an angle of 90 deg. To account for background fluorescence, the fluorescence properties of Aqua (B. Braun, Melsungen, Germany) have also been measured, confirming that this solvent is not fluorescent at the wavelengths of interest. The fluorescence data were postprocessed with an in-house MATLAB algorithm that combined all the emission spectra at several excitation wavelengths into one EEM. 2.4.PharmacokineticsFluorescence emission values were calculated for diluted urine samples in order to assess the pharmacokinetics of the components of OTC vitamin supplements in urine. Since the OTC vitamin supplements have an impact mainly on the second pair of fluorescence peaks (emission wavelength , see below), their maximum intensity was expressed as a percentage of the maximal intensity of the main peak or normalized relative fluorescence intensity. This allowed us to express this data as relative fluorescence intensity, independent of possible day-to-day fluctuations of absolute fluorescence intensity of the urine (due, for instance, to various degrees of dilution of the fluorochromes in the bladder). Baseline values were collected before and after vitamin intake. The baseline values (collected during the week preceding vitamin intake, ) and the of relative fluorescence values collected more than 24 h after the last intake of OTC vitamin supplements () were pooled and averaged to compute the baseline value (the relative fluorescence intensity of the spectral domain where the secondary peaks would be found, but in the absence of OTC vitamin supplements intake) and equaled a numerical value of 6.1 (67% confidence interval: 3.6 to 8.5). This value was subtracted from all other fluorescence intensity values (between 0 and 48 h after OTC vitamin supplements intake). Thus, the values presented in Fig. 3 represent fluorescence intensity corrected for background urine fluorescence at the wavelengths of interest. 3.ResultsDuring our preliminary studies of the BWF fluorescence spectroscopy,10 we observed a typical pattern consisting of a main peak (excitation wavelength: 320 nm, ; emission wavelength: 420 nm, ) assigned to 4-pyridoxic acid and sometimes of a pair of secondary peaks (excitation wavelength: 370 nm/emission wavelength: 525 nm; excitation wavelength: 450 nm/emission wavelength: 525 nm) of identical relative intensities. This pair of secondary peaks can be attributable to riboflavin (vitamin B2).10 We also observed a significant interpatient fluctuation of fluorescence intensity (up to four-fold for the main peak and 5% to 20% relative to the main peak for the pair of secondary peaks). Figure 2 gives the typical EEM of diluted urine from volunteers. In Fig. 2(a), one can see the EEM of the diluted urine of a volunteer consuming a normal, vitamin supplement-free diet. Figure 2(b) gives the same EEM of the diluted urine of the same volunteer consuming a diet supplemented with the OTC vitamin supplement mentioned above. It is very clear from the figure that the spectral properties of the diluted urine change drastically if the volunteer has absorbed this OTC vitamin supplement in addition to his/her normal diet. Fig. 2(a) Fluorescence excitation–emission matrix of the diluted urine of a volunteer consuming a normal diet and (b) a normal diet supplemented with an over-the-counter (OTC) vitamin supplement. 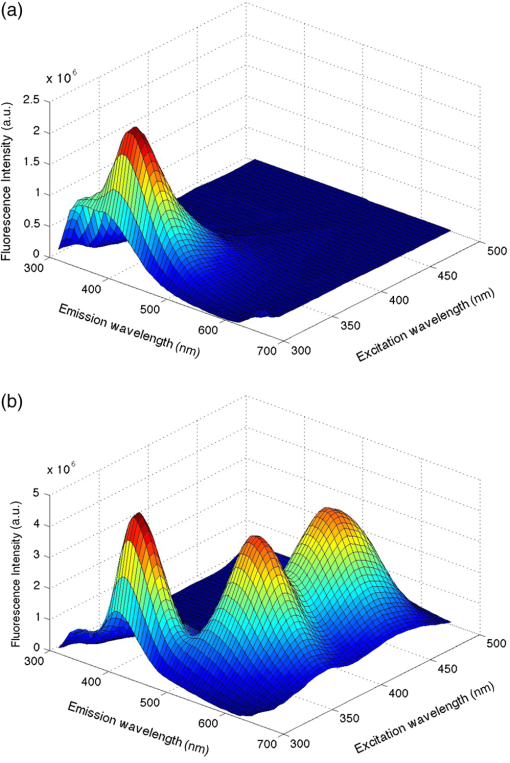 Figure 3 shows the pharmacokinetics of the secondary peaks in healthy volunteers, expressed in hours relative to the daily OTC vitamin supplement intake (set at ). The baseline value (computed from the average of all normalized relative fluorescence intensity values prior to vitamin intake and measured more than 48 h after vitamin intake) is subtracted from the secondary peaks’ fluorescence intensity. The fluorescence intensity of the secondary peaks reaches a maximum within 8 to 10 h after vitamin intake. It then decreases in the half-day that follows, reaching values close to baseline level after . Based on Fig. 3, it is clear that the intake of even one tablet of OTC vitamin supplement generates urine fluorescence intensities above the threshold that represents a macroscopic disturbance for the operator and seriously deteriorates the quality of FC images. Fig. 3Normalized fluorescence intensity of the secondary fluorescence peaks relative to the fluorescence intensity of the main peak expressed in hours relative to the daily OTC vitamin supplement intake (set at , six healthy volunteers, black diamonds) or 48 h after the intake of the last tablet (white circles). 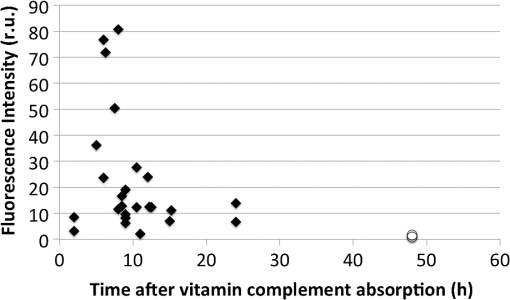 4.DiscussionUrine is a complex biological fluid whose components, if they are fluorescent, can be detrimental to FC. Our goal was to shed some light on one of the possible origins of some of the fluorophores contained in urine to minimize the FC perturbations resulting from urine fluorescence. This information will also help to issue recommendations to the clinicians in the days leading to a FC. Generally speaking, it should be noted that due to the complexity of urine as a mixture, there is no straightforward way to assign its overall fluorescence to a specific molecule since many fluorophores contained in urine have spectral properties that overlap with each other.8–10 We have deliberately chosen to approach this study as a way to improve the FC itself and facilitate the work of the clinicians. While we started by analyzing urine from volunteers, our initial conclusions should allow us to transfer our research to clinical applications with minor adjustments only. This should make it applicable to the reality of clinical settings, with a very direct benefit for patients. We have shown that the OTC vitamin supplement that we studied generates additional fluorescence of urine in the form of secondary fluorescence peaks (excitation wavelength: 370 nm/emission wavelength: 525 nm; excitation wavelength: 450 nm/emission wavelength: 525 nm), which are otherwise very weak in volunteers who did not ingest vitamin supplements. This additional fluorescence is excited and detected by all systems commercially available for FC. We have also demonstrated that urine is back to its baseline fluorescence properties days after the intake of the last OTC vitamin supplement tablet. This means that an optimal preparation for an FC would include discontinuing OTC vitamin supplement at least 2 days before FC, as well as limiting drinks and foods containing substances likely to be metabolized into fluorescent moieties. This is useful information both for the case of outpatients (numerous people ingest OTC vitamin supplements), or for hospitalized patients (many of whom receive supplements of vitamins during their stay at hospital). Additionally, a prior study showed that the fluorescence of the main emission peak is most likely due to 4-pyridoxic acid (with a fluorescence peak at (317 nm/420 nm) (excitation wavelength/emission wavelength), a catabolite of vitamin B6 known to be excreted in urine.13–17 This fluorescence is constantly present, even in the absence of vitamin supplements. After ingestion of the OTC vitamin supplement, its intensity is increased in most volunteers, albeit to a lesser extent than the fluorescence intensity of the secondary peaks (which indicates that other molecules are involved). However, its impact on FC can be minimized by carefully improving the spectral properties of the instruments used to conduct FC and by avoiding any unnecessary exposure of the patient to vitamin B6.10 Our study draws useful conclusions for the optimization of the precystoscopy diet. It is clear that, apart from translating our study into clinical settings, it would be useful to expand it to include not only one OTC vitamin supplement, but several similar cocktails, and to narrow down more precisely which food items should not be consumed during the days leading to an FC. It is noteworthy that the contributions of some food contents, drinks and vitamin supplements to the fluorescence of the diluted urine of volunteers are virtually unexplored and thus of high interest. Our study is preliminary in nature and does not involve a very wide group of volunteers. Our idea was to remove one source of suboptimal images during FC with a simple directive to patients about to undergo an FC. Since this population of patients must undergo the exam for early cancer detection, it is a clear benefit to all that the FC yields images of the highest possible quality. Much is known about substances that color urine. A complete review of the literature on this topic is outside the scope of this study, but one can mention: Urobilin, which is at the origin of the pale yellow color of normal urine;18 carrots and vitamin A, which color the urine orange;18–20 the vitamins of the B group, which color urine bright yellow or orange;21 beets, rhubarb, or blackberries, and more generally, the food items containing betacyanin components, which color urine pinkish;18,22 asparagus, which colors urine green;22 and some food dyes such as chlorophyll or thymol, which color the urine green.18,22 An entirely different matter, however, is the issue of urine fluorescence. As observed in Fig. 1, urine filling the bladder during an FC can itself be fluorescent [Fig. 1(b)] and can screen the fluorescence light emitted by the lesions of interest [Fig. 1(c)]. This is to be compared with conditions of good intravesical visibility [Fig. 1(a)]. According to Leiner et al.,9 the blue–green urine fluorescence is associated with the presence of fluorophores, such as 4-pyridoxic acid, 3-hydroxynthranilic acid, xanthine, and neopterin.9,16,23 Leiner et al.9 also observed additional peaks and they attributed them to the presence of riboflavin or one of its urinary metabolites or a combination thereof. This is in line with our results and corroborates our suggestion that patients undergoing an FC for early cancer detection should by all means avoid vitamin supplements. In our group of volunteers, the pharmacokinetics of vitamin supplements showed a clear fluorescence intensity increase 8 to 10 h after ingestion of the vitamin supplement. A rapid decrease was observed in the half-day following the maximal fluorescence intensity, indicating that more than 50% of the fluorescent vitamins and their fluorescent metabolites were excreted in the 12 h following the ingestion of the vitamin supplement. This is in line with the common sense underlining the prescription to take one tablet/day and validated our assumption that fluorescence intensity would return to values close to baseline values within 24 h. It is interesting to note that our results seem to indicate that although the decrease of urine relative fluorescence intensity is fast after vitamin supplement ingestion, the urine relative fluorescence intensity does not completely return to baseline values until ~2 days following the tapering of vitamin supplement ingestion. This should emphasize the necessity to analyze the diet of patients undergoing an FC in detail, including perhaps foodstuffs or drinks known to contain the vitamins considered here. Most OTC vitamin supplements contain a large range of molecules and minerals. Some of them may contribute to urine color, but few contribute to urine fluorescence. These are mainly some of the vitamins in the B group (B2, B6, B12).13,14,24 By restricting subsequent investigations to these molecules and by corroborating this restriction with our results showing clearly that the fluorescence of these molecules can be detrimental at the wavelengths of interest for FC, one could compensate for the level of detail necessary to avoid detrimental urine background fluorescence. This simple approach would be a major improvement for the clinicians preparing patients for a diagnostic FC. 5.ConclusionIn order to obtain the best possible fluorescence images during diagnostic FC, detrimental background fluorescence should be minimized. Urine, secreted during the FC, contains a number of fluorophores and contributes to this detrimental background fluorescence. This is at least in part due to the patient’s diet and metabolism. One source of such fluorophores is the B group of vitamins, in particular, vitamins B2, B6, and B12, contained in OTC vitamin supplements, such as Berocca (15 mg, 10 mg, and , respectively). If the findings of our study were to be extrapolated to clinical settings, one could conclude that it is likely best that the intake of OTC vitamin supplements be avoided during the week preceding an FC. AcknowledgmentsThis work was supported by the Swiss National Science Foundation (Grant Nos. 205320-130518, 205320_147141/1, and CR32I3_150271/1) and funded in part by the J. Jacobi Trust. The authors also gratefully acknowledge the financial support of Ipsen (France). ReferencesM. Babjuk et al.,
“Guidelines on non-muscle-invasive bladder cancer (Ta, T1 and CIS),”
Eur. Assoc. Urol., 12
(2015). Google Scholar
I. Kausch et al.,
“Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies,”
Eur. Urol., 57 595
–606
(2010). http://dx.doi.org/10.1016/j.eururo.2009.11.041 EUURAV 0302-2838 Google Scholar
M. Burger et al.,
“Epidemiology and risk factors of urothelial bladder cancer,”
Eur. Urol., 63 234
–241
(2013). http://dx.doi.org/10.1016/j.eururo.2012.07.033 Google Scholar
H. Barton Grossman et al.,
“Long-term decrease in bladder cancer recurrence with hexaminolevulinate enabled fluorescence cystoscopy,”
J. Urol., 188 58
–62
(2012). http://dx.doi.org/10.1016/j.juro.2012.03.007 Google Scholar
G. Wagnières et al.,
“Detection of bladder cancer by fluorescence cystoscopy: from bench to bedside - the hexvix story,”
Handbook of Photomedicine, 411
–426 CRC Press (Taylor & Francis), Boca Raton, Florida
(2013). Google Scholar
D. Jocham, H. Stepp and R. Waidelich,
“Photodynamic diagnosis in urology: state-of-the-art,”
Eur. Urol., 53 1138
–1150
(2008). http://dx.doi.org/10.1016/j.eururo.2007.11.048 EUURAV 0302-2838 Google Scholar
J. A. Witjes and J. Douglass,
“The role of hexaminolevulinate fluorescence cystoscopy in bladder cancer,”
Nat. Clin. Pract. Urol., 4
(10), 542
–549
(2007). http://dx.doi.org/10.1038/ncpuro0917 Google Scholar
J. Kusnir et al.,
“Concentration matrices-solutions for fluorescence definition of urine,”
Anal. Lett., 38 1559
–1567
(2005). http://dx.doi.org/10.1081/AL-200065787 Google Scholar
M. J. P. Leiner, M. R. Hubmann and O. S. Wolfbeis,
“The total fluorescence of human urine,”
Anal. Chim. Acta, 198 13
–23
(1987). http://dx.doi.org/1016/S0003-2670(00)85002-3 ACACAM 0003-2670 Google Scholar
C. Martoccia et al.,
“Optical spectroscopy of the bladder washout fluid to optimize fluorescence cystoscopy with Hexvix®,”
J. Biomed. Opt., 19
(9), 097002
(2014). http://dx.doi.org/10.1117/1.JBO.19.9.097002JBOPFO1083-3668 Google Scholar
R. Rajasekaran et al.,
“Synchronous luminescence spectroscopic characterization of urine of normal subjects and cancer patients,”
J. Fluoresc., 24 1199
–1205
(2014). http://dx.doi.org/10.1007/s10895-014-1401-4 Google Scholar
M. Mengin, Google Scholar
T. Tsuji et al.,
“Urinary excretion of vitamin B1, B2, B6, niacin, pantothenic acid, folate, and vitamin C correlates with dietary intakes of free-living elderly, female Japanese,”
Nutr. Res., 30
(3), 171
–178
(2010). http://dx.doi.org/10.1016/j.nutres.2010.02.001 Google Scholar
G. F. Combs, The Vitamins: Fundamental Aspects in Nutrition and Health, 2nd ed.Academic Press, San Diego
(1998).). Google Scholar
C. Bueno, J. Guerrero and M. V. Encinas,
“Spectroscopic properties of 4-pyridoxic acid as a function of pH and solvent,”
Helv. Chim. Acta, 87 940
–948
(2004). http://dx.doi.org/10.1002/hlca.200490087 HCACAV 0018-019X Google Scholar
K. Dubayová, J. Kusnir and L. Podracká,
“Diagnostic monitoring of urine by means of synchronous fluorescence spectrum,”
J. Biochem. Biophys. Methods, 55 111
–119
(2003). http://dx.doi.org/10.1016/S0165-022X(03)00031-9 JBBMDG 0165-022X Google Scholar
S. G. Schulman,
“Molecular luminescence spectroscopy, methods and applications: part 1,”
Chemical Analysis, A Series of Monographs On Analytical Chemistry And its Applications, 77 187 John Wiley & Sons, Hoboken, New Jersey
(1985). Google Scholar
J. R. Raymond and W. E. Yarger,
“Abnormal urine color: differential diagnosis,”
South. Med. J., 81
(7), 837
–841
(1988). http://dx.doi.org/10.1097/00007611-198807000-00008 SMJOAV 0038-4348 Google Scholar
A. Kulberg,
“Urinalysis and urine culture,”
Top. Emerg. Med., 5
(1), 47
–61
(1983). Google Scholar
N. Risborough Lawrie, T. Moore and K. R. Rajagopal,
“The excretion of vitamin A in urine,”
Biochem. J., 35
(7), 825
–836
(1941). Google Scholar
R. G. Tucker, O. Mickelsen and A. Keys,
“The influence of sleep, work, diuresis, heat, acute starvation, thiamine intake and bed rest on human riboflavin excretion,”
J. Nutr., 72 251
–261
(1960). Google Scholar
S. C. Mitchell,
“Food idiosyncrasies: beetroot and asparagus,”
Drug Metab. Dispos., 29
(4), 539
–543
(2001). DMDSAI 0090-9556 Google Scholar
S. M. Perinchery et al.,
“The potential of autofluorescence spectroscopy to detect human urinary tract infection,”
Talanta, 82 912
–917
(2010). http://dx.doi.org/10.1016/j.talanta.2010.05.049 TLNTA2 0039-9140 Google Scholar
T. Fukuwatari et al.,
“Urinary excretion of vitamin B12 depends on urine volume in Japanese female university students and elderly,”
Nutr. Res., 29
(12), 839
–845
(2009). http://dx.doi.org/10.1016/j.nutres.2009.10.008 Google Scholar
Biography |

