|
|
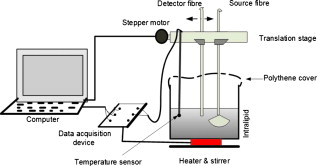
1.IntroductionAccurate knowledge of the optical properties of turbid media, such as tissue, is important in many clinical applications that use light for diagnosis and treatment. The optical properties depend on the physiological state of the tissue and are influenced by many parameters. One such parameter is temperature, which can substantially alter measured optical properties. The effect can be quantified as a temperature coefficient, the rate of increase (or decrease) in optical absorption and scattering coefficients with temperature. Temperature-induced change in the optical properties can, for example, limit the application of photodynamic therapy.1 Other applications include the estimation of temperature from the change in optical, property measured. Temperature can be estimated from changes in the absorbance2 however, in turbid media, variation in scatter introduces an uncertainty in the path length, making it difficult to reliably estimate temperature from optical extinction coefficients. Uncoupling the effect of scattering on absorption may reduce this obscurity, offering the potential for accurate measurements of temperature in turbid solutions. Kelly 3 measured the near-infrared (NIR) spectra of chicken, bovine, and porcine tissues from with a spectrophotometer and reported that the measurements can predict tissue temperature with a standard error less than . Laufer 4 measured the change in optical properties of human dermis and subdermis from . They observed positive and negative temperature coefficients of scattering for dermis and subdermis, respectively, but no change in absorption with temperature. A common phantom to simulate optical properties of tissue is Intralipid. Temperature-induced changes in the optical properties of Intralipid have been described previously. Kakuta 2 reported the temperature dependence of absorbance at using a spectrophotometer and proposed a method to measure the temperature of turbid aqueous solutions, but they did not recover the absorption coefficient. McGlone 5 determined the temperature dependence of absorption and reduced scattering coefficients of Intralipid using continuous wave photon migration measurements from . The authors relied on measurement of attenuation close to the source to separate scattering and absorption. The changes in absorption coefficients due to temperature were attributed to water,6, 7 the main absorbing species in Intralipid.8, 9 Kakuta found a discrepancy in absorption between heating and cooling cycle measurements and linked it to irreversible changes in the scattering properties of the micelles. McGlone observed a lack of repeatability, during heating and cooling, in temperature-induced changes in the reduced scattering properties. The frequency-domain technique uncouples absorption from scattering10, 11 when determining the optical properties of turbid media, which potentially allows more accurate recovery of optical coefficients.12 Our work is a preliminary step toward monitoring these subtle changes. We have investigated the optical properties of a tissue-simulating phantom and present, for the first time, frequency-domain measurements of the temperature dependence of the absorption and reduced scattering coefficients of Intralipid in the wavelength range from . We have used a 1.8% (w/w) solution of Intralipid as the sample to simulate the reduced scattering properties of human brain and breast tissues.13 Measurements were made in the physiologically relevant temperature range of while heating and cooling the liquid. 2.MethodOptical properties can be determined by measuring the amplitude attenuation and phase shift of intensity-modulated light passing through a turbid medium.14 The propagation of intensity-modulated light through turbid media has been extensively discussed in the literature.15, 16, 17, 18 We employed the multidistance method, where the amplitude and phase of a photon density wave is measured at different separations from the source. An outline of our experimental setup is shown in Fig. 1 and has been described in detail previously.19 Using this configuration, we measured absorption to an agreement with reported literature of better than 10% in the presence of reduced scattering coefficients between 10 and . Briefly, the instrumentation employed a tunable Ti: Sapphire laser as the light source, which was intensity modulated by an acousto-optic modulator. A lock-in amplifier in conjunction with an avalanche photodiode (APD) module constituted a phase-sensitive detection system. Optical fibers, suspended from a translation stage in the middle of the Intralipid solution, were used to couple the light into and out of the medium. The temperature of the medium was maintained using a closed-loop control system incorporating a heated plate (VELP Scintifica, Italy) equipped with a magnetic stirrer and a semiconductor temperature sensor (LM35, National Semiconductor, Santa Clara, California) immersed in the solution. The temperature sensor was located so that it would not affect the photon density wave. The temperature sensor and the solution heater were connected via a data acquisition device (U 12, LabJack Corporation, Lakewood, Colorado) to a computer and controlled by a Labview (National Instruments, Austin, Texas) program. A loose polythene cover minimized water evaporation during the experiment. A 1.8% (w/w) solution of Intralipid was obtained by diluting of Intralipid-20% (Pharmaco, New Zealand) in of distilled water. Amplitude and phase measurements were initially collected, at intervals, between 710 and at three different set-point temperatures (30, 35, and —the “low-temperature resolution” data) as the distance between the source and detector fibers was increased from ( steps). At each stage-position, five amplitude and phase measurements were recorded to estimate the errors in our estimated absorption and reduced scattering coefficients. The temperature control system was allowed to stabilize for at least before collecting each set of measurements. Additional amplitude and phase measurements—the “high-resolution” data—were made at 740, 800, and at room temperature , then as the solution was first slowly heated (over ) from , then again as it cooled (over ), and finally again at room temperature. These measurements were collected every between over the heating and cooling regimes. Replicate heating and cooling measurements were collected at and with similar solutions prepared, on different days; a single set of measurements was recorded at . Last, optical measurements were recorded when the solution reached after a cooling cycle, then again at the next day. As Intralipid is known to decay over time,20 a fresh solution was prepared for each set of measurements. 3.ResultsFigure 2 shows the absorption coefficient of Intralipid, calculated from the amplitude and phase measurements, over the wavelength range measured at the three different temperatures. The absorption coefficient from previous measurements made by us at is included.19 The error bars at each measurement illustrate the measurement uncertainty ( standard deviation). The uncertainty in the optical coefficients was estimated from the replicate error in phase and amplitude measurements through error propagation.21 Arrows on the plot show the trend in absorption coefficient with temperature. The absorption coefficient increases around 740 and and decreases around . Fig. 2Absorption coefficients measured at (triangle),19 (hexagram), (circle), and (diamond) plotted against wavelength. The changes in absorption around 740, 800, and are indicated by arrows. 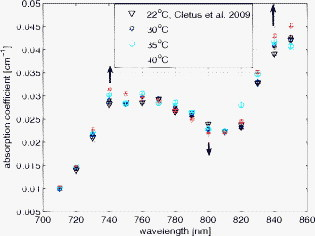 The reduced scattering coefficient, again calculated from the amplitude and phase measurements, is illustrated in Fig. 3 for the three temperatures measured in this study and at (Ref. 19). As predicted by Mie theory,22 the reduced scattering coefficient decreases, nearly linearly, as wavelength increases. Moreover, these measurements show that the reduced scattering coefficient decreases as temperature increases. Fig. 3Reduced scattering coefficient measured at different temperatures plotted against wavelength. Linear, least-squares fits at (red) and (black) show the trends (dashed lines). (Color online only.) 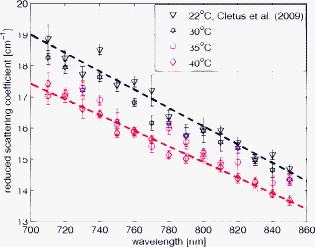 The temperature trend is more clearly illustrated for the absorption and reduced scattering coefficients using the data measured continuously as the sample was heated and cooled (Fig. 4 and Fig. 5 , respectively). Each plot illustrates an approximately linear relationship between the optical coefficient and temperature at 740 and for two replicate measurements during heating and cooling of the sample. Results at are included for comparison from earlier work.19 Each data point between 30 and represents the average of five measurements with one standard deviation error bars estimated by propagating the noise in the amplitude and phase measurements through the calculations for the optical coefficients. Fig. 4Absorption coefficient at 740 and plotted against temperature. The red circles correspond to the heating cycle data, and the blue asterisks to the cooling cycle. The solid line is a least-squares fit to all the data, and the dashed lines indicate 68% confidence interval. The error bars give standard measurement error. (Color online only.) 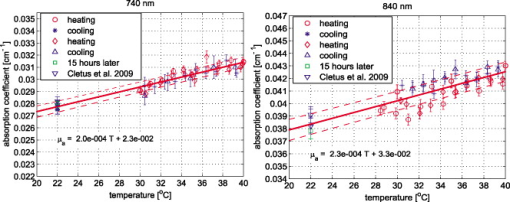 Fig. 5Reduced scattering coefficients at 740 and plotted against temperature. The red circles indicate heating, and the blue asterisks cooling cycles. The solid lines are least-squares fits to heating and cooling data. The error bars and confidence interval show standard error. (Color online only.) 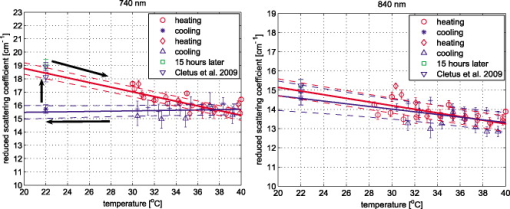 As Fig. 4 shows, the absorption coefficient increases with temperature at both 740 and . This is consistent with the change in absorption coefficient of pure water,7 the main absorbing species in Intralipid.8 Lipid, the other absorbing species in Intralipid, has an absorption coefficient of less than but is present only at 1.8% by volume, so its contribution will be negligible.9 The absorption coefficients over the heating and cooling period match well at . However, at , absorption was generally higher during the cooling period. This may be due to a variation in dilution across the different batches of Intralipid. A temperature coefficient, , has been estimated using a linear, least-squares fit to the measurements made in the heating and cooling periods. The discrepancy between heating and cooling at had no significant effect on the slope, so data from the two periods has been combined in all cases. These measurements are in good agreement with results collected at both during this work and in previous work.19 Generally, the reduced scattering coefficient decreased as temperature increased at 740 and (Fig. 5). However, at , a hysteresis was observed whereby the reduced scattering coefficient decreased during heating yet remained reasonably constant during cooling, even down to . This behavior was observed in both replicates but was not a result of the self cooling, as the measurement time for a single set (a detector fiber scan from ) is about and the change in temperature in this interval is only of the order of . Earlier data at and a subsequent measurement at (green square) on the following day ( later), indicate that the scattering coefficient recovers to its original value eventually. This behavior was observed in both replicates at , but at , although the slope is slightly lower during the cooling period, no significant difference was observed between the heating and cooling periods. Kakuta 2 and McGlone 5 also observed different behavior in their measurements during heating and cooling. Kakuta observed discrepancy in their absorbance measurement and postulated that it may be due to the permanent structural changes in the micelles during heating. McGlone also observed different scattering behavior during heating and cooling. Because of this hysteresis, the temperature coefficient, , for the scattering coefficient was estimated using only data from the heating period, with a linear least-squares fit. 4.DiscussionA positive temperature coefficient of absorption of at and at was calculated from the data plotted in Fig. 4. At (data not shown), we calculated a temperature coefficient of . The near-infrared absorption features arise from overtones and combination bands of the vibrations of the water molecule.23 At , the main contribution is a combination of symmetric and asymmetric stretch and bending vibrations. The absorption peak is caused by symmetric and asymmetric stretch vibrations. The sensitivity of water absorption to temperature arises from changes to the strength of the hydrogen bonds and microscopic changes in the structure of the water.7, 24 There is some disagreement in the literature over the precise value of water’s near-infrared temperature coefficient. Our results at 740 and are in good agreement with those reported by Hollis25, 26 for water but are 40% higher than the temperature coefficient of water reported by Langford (Fig. 6 ).7 The temperature coefficient of absorption for Intralipid measured by McGlone 5 are about 50% lower than our results. At , our result is more consistent with the data reported by Langford 7 and McGlone, 5 underestimating Hollis’ observation by 75%. Fig. 6Temperature coefficient of absorption for 1.8% solution of Intralipid plotted against wavelength. The temperature coefficient for pure water reported by Hollis and Langford 7, 25, 26 and the temperature coefficient for Intralipid (1%) reported by McGlone 5 are also included. Error bars indicate 68% confidence interval. 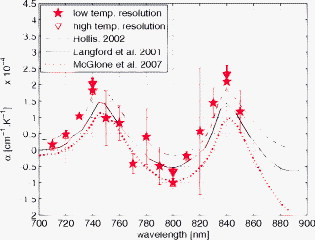 The temperature coefficient of Intralipid absorption was estimated in steps between 710 and using the data plotted in Fig. 3. At most wavelengths, the temperature coefficient was estimated from absorption measured at 22, 30, 35, and (squares). At 740, 800, and , the results, obtained from high-temperature resolution measurements, discussed earlier are plotted (triangles). The error bars indicate 68% confidence interval of the regression values. Large errors—at , for example—occur when there is a significant variation in laser power during a measurement. Overall, our results agree in magnitude and spectral shape with values reported in the literature. The prediction intervals in Fig. 4 give an indication of the temperature sensitivity of our setup. The minimum temperature change observable within the error limits is and at 740 and , respectively. This is probably insufficient for applications requiring precision measurement. Moreover, the presence of other absorbing species, such as hemoglobin, in biological samples will likely demand even greater accuracy. However, we believe that the largest source of error lies in the precision of the phase measurements and the short-term stability of the source. We believe that more precise temperature measurements can be achieved by including active feedback to control the amplitude stability of our laser source. The temperature coefficient of the reduced scattering coefficient was estimated, as for absorption, using linear regression. However, as we found a significant difference in scattering behavior during heating and cooling, only heating data was used. The results are plotted in Fig. 7 . At most wavelengths, the temperature coefficient was estimated from reduced scattering measured at 22, 30, 35, and (squares); at 740, 800, and , approximately 10 measurements between 30 and , as well as were used (triangles). A linear regression line through the reduced scattering temperature coefficient shows a general decrease in temperature sensitivity as wavelength increases. Fig. 7Temperature coefficient for reduced scattering coefficient ( standard error) plotted against wavelength. The red line shows a linear fit to the temperature coefficient, and the dotted lines indicate 68% confidence intervals. The dashed (green) line shows the calculated temperature coefficient. (Color online only.) 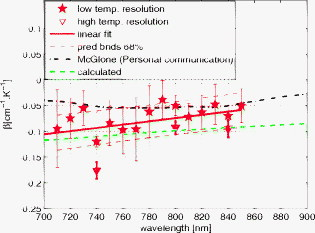 The scattering coefficient of a turbid media is affected by temperature in two ways. First, as temperature increases, the volume of the medium expands, diluting the scattering effect.5 However, more significantly, the refractive index of water also decreases with temperature,27 leading to a greater mismatch between the scattering particles and the absorbing medium. We have modeled the temperature coefficient of the reduced scattering coefficient of Intralipid based on Mie scattering and changes of the refractive index with temperature. We used published particle-size distribution data for Intralipid9 and assumed a refractive index temperature sensitivity of , as that value is typical of the major fatty acids components of soya bean oil and similar derivatives.28 The result is included in Fig. 7 as a green dashed line for comparison. McGlone measured temperature coefficients for scattering in a 1.2% Intralipid solution by continuous wave measurements. Their data, scaled to 1.8%, are illustrated as well. Within the large uncertainty, our measurements agree with theory and other measurements. 5.ConclusionWe have used frequency-domain, photon-migration spectroscopy to separately measure absorption and reduced scattering temperature coefficients in a turbid medium for the first time. The optical temperature coefficients of the liquid, tissue-simulating phantom 1.8% Intralipid were estimated from in the physiologically relevant temperature range. At 740, 800, and (key wavelengths in the water absorption temperature coefficient), we measured absorption temperature coefficients of , , and and reduced scattering temperature coefficients of , , and , respectively. The temperature coefficients observed for absorption closely follow the published results for water, as expected. The temperature coefficients observed for reduced scattering exhibited a general decrease as wavelength increased, consistent with predictions made by Mie theory and literature. We observed hysteresis behavior in the reduced scattering measurements made at that did not appear to occur at higher wavelengths. The reduced scattering coefficient decreased with temperature but did not increase again as the solution was cooled until sometime later. This hysteresis did not affect the absorption measurements. This result suggests that similar observations of this behavior reported in extinction measurements are caused by changes in the scattering micelles. These results suggest that temperature measurement, with a precision of , in turbid media is feasible based on the absorption coefficient’s temperature coefficient. However, an improvement in precision will be required for many applications. AcknowledgmentsWe acknowledge financial support from the University of Waikato and the Foundation for Research Science and Technology. ReferencesJ. Svensson,
A. Johansson,
K. Svanberg, and
S. Andersson-Engels,
“Tissue temperature monitoring during interstitial photodynamic therapy,”
Proc. SPIE, 5698 126
–136
(2005). https://doi.org/10.1117/12.588485 0277-786X Google Scholar
N. Kakuta,
H. Arimoto,
H. Momoki,
F. Li, and
Y. Yamada,
“Temperature measurements of turbid aqueous solutions using near-infrared spectroscopy,”
Appl. Opt., 47
(13), 2227
–2233
(2008). https://doi.org/10.1364/AO.47.002227 0003-6935 Google Scholar
J. J. Kelly,
K. A. Kelly, and
C. H. Barlow,
“Tissue temperature by near-infrared spectroscopy,”
Proc. SPIE, 2389 818
–828
(1995). https://doi.org/10.1117/12.210025 0277-786X Google Scholar
J. Laufer,
R. Simpson,
M. Kohl,
M. Essenpreis, and
M. Cope,
“Effect of temperature on the optical properties of ex vivo human dermis and subdermis,”
Phys. Med. Biol., 43
(9), 2479
–2489
(1998). https://doi.org/10.1088/0031-9155/43/9/004 0031-9155 Google Scholar
V. A. McGlone,
P. Martinsen,
R. Künnemeyer,
R. Jordan, and
B. Cletus,
“Measuring optical temperature coefficients of Intralipid,”
Phys. Med. Biol., 52
(9), 2367
–2378
(2007). https://doi.org/10.1088/0031-9155/52/9/003 0031-9155 Google Scholar
J. R. Collins,
“Change in the infra-red absorption spectrum of water with temperature,”
Phys. Rev., 26
(6), 771
–779
(1925). https://doi.org/10.1103/PhysRev.26.771 0031-899X Google Scholar
V. S. Langford,
A. J. McKinley, and
T. I. Quickenden,
“Temperature dependence of the visible-near-infrared absorption spectrum of liquid water,”
J. Phys. Chem. A, 105
(39), 8916
–8921
(2001). https://doi.org/10.1021/jp010093m 1089-5639 Google Scholar
S. T. Flock,
S. L. Jacques,
B. C. Wilson,
W. M. Star, and
M. J. van Gemert,
“Optical properties of Intralipid: a phantom medium for light propagation studies,”
Lasers Surg. Med., 12
(5), 510
–519
(1992). https://doi.org/10.1002/lsm.1900120510 0196-8092 Google Scholar
H. J. van Staveren,
C. J. M. Moes,
J. van Marie,
S. A. Prahl, and
M. J. C. van Gemert,
“Light scattering in Intralipid-10% in the wavelength range of ,”
Appl. Opt., 30
(31), 4507
–4514
(1991). https://doi.org/10.1364/AO.30.004507 0003-6935 Google Scholar
A. E. Cerussi,
A. J. Berger,
F. Bevilacqua,
N. Shah,
D. Jakubowski,
J. Butler,
R. F. Holcombe, and
B. J. Tromberg,
“Sources of absorption and scattering contrast for near-infrared optical mammography,”
Acad. Radiol., 8
(3), 211
–218
(2001). https://doi.org/10.1016/S1076-6332(03)80529-9 1076-6332 Google Scholar
J. B. Fishkin,
P. T. C. So,
A. E. Cerussi,
S. Fantini,
M. A. Franceschini, and
E. Gratton,
“Frequency-domain method for measuring spectral properties in multiple-scattering media: methemoglobin absorption spectrum in a tissuelike phantom,”
Appl. Opt., 34
(7), 1143
–1155
(1995). https://doi.org/10.1364/AO.34.001143 0003-6935 Google Scholar
M. Gerken and
G. W. Faris,
“High-precision frequency-domain measurements of the optical properties of turbid media,”
Opt. Lett., 24
(14), 930
–932
(1999). https://doi.org/10.1364/OL.24.000930 0146-9592 Google Scholar
W. F. Cheong,
S. A. Prahl, and
A. J. Welch,
“A review of the optical properties of biological tissues,”
IEEE J. Quantum Electron., 26
(12), 2166
–2185
(1990). https://doi.org/10.1109/3.64354 0018-9197 Google Scholar
S. Fantini,
M. A. Franceschini,
J. B. Fishkin,
B. Barbieri, and
E. Gratton,
“Quantitative determination of the absorption spectra of chromophores in strongly scattering media: a light-emitting-diode based technique,”
Appl. Opt., 33
(22), 5204
–5213
(1994). https://doi.org/10.1364/AO.33.005204 0003-6935 Google Scholar
J. B. Fishkin,
E. Gratton,
M. J. van de Ven, and
W. W. Mantulin,
“Diffusion of intensity modulated near-infrared light in turbid media,”
Proc. SPIE, 1431 122
–135
(1991). https://doi.org/10.1117/12.44184 0277-786X Google Scholar
J. B. Fishkin and
E. Gratton,
“Propagation of photon-density waves in strongly scattering media containing an absorbing semi-infinite plane bounded by a straight edge,”
J. Opt. Soc. Am. A, 10
(1), 127
–140
(1993). https://doi.org/10.1364/JOSAA.10.000127 0740-3232 Google Scholar
B. J. Tromberg,
L. O. Svaasand,
T. T. Tsay, and
R. C. Haskell,
“Properties of photon density waves in multiple-scattering media,”
Appl. Opt., 32
(4), 607
–616
(1993). https://doi.org/10.1364/AO.32.000607 0003-6935 Google Scholar
R. C. Haskell,
L. O. Svaasand,
T. T. Tsay,
T. C. Feng,
M. S. McAdams, and
B. J. Tromberg,
“Boundary conditions for the diffusion equation in radiative transfer,”
J. Opt. Soc. Am. A, 11
(10), 2727
–2741
(1994). https://doi.org/10.1364/JOSAA.11.002727 0740-3232 Google Scholar
B. Cletus,
R. Künnemeyer,
P. Martinsen,
A. McGlone, and
R. Jordan,
“Characterizing liquid turbid media by frequency-domain photon migration spectroscopy,”
J. Biomed. Opt., 14
(2), 024041
–024047
(2009). https://doi.org/10.1117/1.3119282 1083-3668 Google Scholar
T. L. Whateley,
G. Steele,
J. Urwin, and
G. A. Smail,
“Particle size stability of Intralipid and mixed total parenteral nutrition mixtures,”
J. Clin. Pharm. Ther., 9
(2), 113
–126
(2008). https://doi.org/10.1111/j.1365-2710.1984.tb01067.x 0269-4727 Google Scholar
J. R. Taylor, An Introduction to Error Analysis: The Study of Uncertainties in Physical Measurements, University Science Books, Sausalito, CA
(1982). Google Scholar
Q. Fu and
W. Sun,
“Mie theory for light scattering by a spherical particle in an absorbing medium,”
Appl. Opt., 40
(9), 1354
–1361
(2001). https://doi.org/10.1364/AO.40.001354 0003-6935 Google Scholar
J. G. Bayly,
V. B. Kartha, and
W. H. Stevens,
“The absorption spectra of liquid phase , HDO and from ,”
Infrared Phys., 3
(4), 211
–222
(1963). https://doi.org/10.1016/0020-0891(63)90026-5 0020-0891 Google Scholar
M. Praprotnik,
D. Janezic, and
J. Mavri,
“Temperature dependence of water vibrational spectrum: a molecular dynamics simulation study,”
J. Phys. Chem. A, 108
(50), 11056
–11062
(2004). https://doi.org/10.1021/jp046158d 1089-5639 Google Scholar
V. S. Hollis,
“Non-invasive monitoring of brain tissue by near-infrared spectroscopy,”
University College London,
(2002). Google Scholar
V. S. Hollis,
T. Binzoni, and
D. T. Delpy,
“Non-invasive monitoring of brain tissue temperature by near-infrared spectroscopy,”
Proc. SPIE, 4250 470
–481
(2001). https://doi.org/10.1117/12.434506 0277-786X Google Scholar
C. H. Cho,
J. Urquidi,
G. I. Gellene, and
G. W. Robinson,
“Mixture model description of the T-, P dependence of the refractive index of water,”
J. Chem. Phys., 114
(7), 3157
–3162
(2001). https://doi.org/10.1063/1.1331571 0021-9606 Google Scholar
M. P. P. Castro,
A. A. Andrade,
R. W. A. Franco,
P. C. M. L. Miranda,
M. Sthel,
H. Vargas,
R. Constantino, and
M. L. Baesso,
“Thermal properties measurements in biodiesel oils using photothermal techniques,”
Chem. Phys. Lett., 411
(1), 18
–22
(2005). https://doi.org/10.1016/j.cplett.2005.06.003 0009-2614 Google Scholar
|