|
|
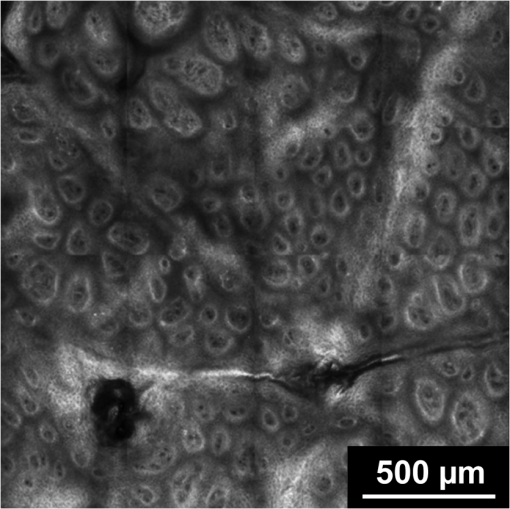
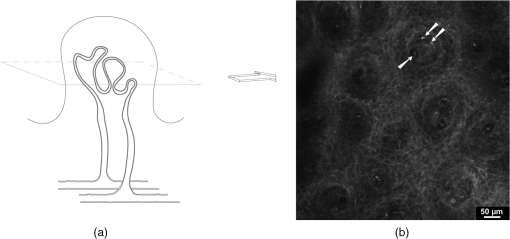
1.IntroductionPsoriasis is a chronic recurring inflammatory skin disease associated with the development of erythematosquamous patches and plaques and a disturbed skin barrier function.1,2 A prevalence of 0.6%–4.8%3 has been reported in the worldwide population. Although, a number of etiologic aspects have been described in detail, several pathogenetic mechanisms still remain unknown. It has been mentioned that the underlying inflammatory reaction of psoriasis dominated by TH 1 cells is followed by epidermal hyperproliferation, disturbed differentiation, and structural changes in the papillary capillaries.4 The blood circulation system of the skin is composed of two horizontally arranged plexi, located parallel to the skin surface. The deep dermal plexus, lying on the border to the subcutis, sends arterioles to the upper plexus lying within the zone of the dermal-epidermal junction. The upper plexus supplies every papilla with a hairpin-shaped capillary loop.5–7 The loops observed in the capillary vessels of psoriatic plaques have been described as elongated, widened, and tortuous, especially in the papillary dermis.8 There has been much discussion in recent years about whether psoriasis is primarily an epidermal disease that is secondarily accompanied by dermal and vascular changes, or whether the disease originates from the dermis and blood vessels and secondarily provokes a hyperproliferation of the epidermis. An important and most likely active role in the pathogenesis of psoriasis has been described to be attributed to changes in blood vessels.9 Light-microscopic investigations made during the treatment of psoriatic lesions led to the conclusion that dilatations of the capillary vessels of the dermal papillae persisting after complete healing of the lesions facilitate recurrence in loco.10 Electron-microscopic investigations showed dermal capillary loops in affected psoriatic skin to be venous capillaries.8 Following dermatological therapy using photochemotherapy with oral psoralen and UVA irradiation (PUVA) and UVB therapies, venous blood capillaries reverted to become arterial capillaries like those existing in healthy skin. This fact draws attention to the important role of the dermal blood vessels and their changes during the development, progress, and healing of psoriasis.7,8 Confocal laser-scanning microscopy (CLSM) represents a promising method for noninvasive in vivo evaluation of capillaries that has been applied to elucidate structural, functional, and morphological skin characteristics.11–15 CLSM has previously been used for evaluation of psoriasis, whereby CLSM features of psoriasis have been shown to correlate well with histopathological findings.16,17 It has been proposed that CLSM may be a promising method for noninvasive assessment of erythematosquamous skin diseases with good sensitivity and specificity for psoriasis (89.13% and 95.41%, respectively).18 The state of dermal vasculature may represent a critical factor in predicting the course of this recurring disease. Regarding this matter, previous studies on healthy skin have shown reproducible visualization and measurement of skin capillaries using CLSM.19 Based on these findings, the purpose of this study was to evaluate CLSM as a method for investigating structure and morphology of dermal capillaries in psoriasis patients in comparison to patients with normal skin, which, to our knowledge, has not yet been performed. 2.Materials and Methods2.1.Confocal Laser Scanning MicroscopyFor the investigations in this study, a confocal laser-scanning microscope (VivaScope® 1500, Lucid Inc, Rochester, NY) was used. The measuring device is described in detail elsewhere.20 Briefly, to generate confocal images, a laser beam in the near-infrared range (830 nm) is emitted through an optical system and focused on the area to be investigated. Reflected and backscattered light is returned through a small aperture and then focused onto a photodetector. A thin optical plane or section can be imaged at high resolution and contrast. Sections of human skin as well as cellular details can be visualized in vivo without biopsy and further histological processing or staining. The confocal microscope produces horizontal (en face) skin sections, differing from vertical sections obtained by routine histology. Thereby, images with a vertical resolution of 5 µm and a lateral resolution of 1–3 µm can be analyzed without the administration of exogenous contrast agents. Contrasts in CLSM images are due to variations in refractive indices of tissue structures. Cytoplasm, which has a refractive index similar to that of water, is seen with little contrast, whereas melanin and keratin are seen as highly refractive structures, and their refractive indices function as natural contrast agents.20 2.2.Study ParticipantsThe investigations were carried out on 13 patients who had a longstanding history of chronic plaque psoriasis and were suffering from an acute relapse. None of these patients had undergone systemic or local treatment (except for a keratolytic therapy with salicylates for better optical penetration) at least 2 months before the investigations. Five of the patients were female (median age 51 years, mean 46.6 years), and 8 were male (median age 60 years, mean 55.7 years). A group of 5 volunteers (1 woman, 4 men; median age 28 years, mean 29 years) with healthy skin served as controls. All study participants had skin phototype I–III. We conformed to the Declaration of Helsinki as a statement of ethical principles for medical research involving human subjects. The study was approved by the Ethics Committee of the Charité. 2.3.CLSM Imaging ProtocolBoth groups were investigated at room temperature (20°C–24°C) and after a resting period of at least 5 min. Skin sites for imaging were selected on the lower leg and/or forearm. Single images () were obtained from the center of the lesion from different skin levels starting at the stratum corneum down to the papillary dermis, and partially the upper reticular dermis, at 5-µm intervals. The laser intensity was adjusted manually to achieve images with a good contrast of the dermal structures. At the upper level of the papillary dermis, where the ascending and descending limbs of the capillary loop were visible, many (at least five) single images from every participant were taken by scanning horizontally along the - and -axes parallel to the surface of the skin. Because of individual skin properties, capillary values could not be measured at exactly the same depth of the skin in each participant.21 An earlier observation using CLSM, however, showed constant levels of capillary perimeters in different depths of the skin.19 2.4.CLSM Image AnalysisFor the purpose of this study, we compared the structures of the capillary loops in healthy participants with those in affected skin of psoriasis patients. Morphological differences between the capillary loops in the two groups were mainly identified by following the loop components at a 5-µm depth interval; afterward, the diameters of all clearly identified loop capillaries and dermal papillae were measured. A commercially available software (AutoCAD 2010®, Autodesk Inc., San Rafael, CA) was used to allow precise measurement of the skin structures by means of image calibration. Exact identification and colocalization of measurement points was enabled using the zoom function. For the purpose of this study, we determined the outer diameter of the dermal capillaries by including an area of a thin halo around the visible blood flow, representing the endothelial tube of the capillaries. Similarly, the diameter of the dermal papilla was determined by including the surrounding bright layer composed of melanocytes and basal cells. 2.5.Statistical AnalysisThe diameters of capillaries and papillae were represented as mean standard deviation. The Levene test was used for testing equality of variance. Comparison was made using Student’s test. Statistical analysis was performed using SPSS 18.0 Standard version for Windows (SPSS Inc., Chicago, IL). 3.Results3.1.General FindingsDuring the investigations carried out in healthy volunteers, the dermal layers from the stratum corneum to the dermal papillae have been well represented and easily identified [Fig. 1(a) to 1(c)]. The stratum corneum consisted of 10–20 layers of anucleated corneocytes, with polygonal shape whose arrangement appeared as a homogeneous white surface, owing to the high refractive index of the corneocytes. Further distally, the stratum granulosum was visible with clearly recognizable cell boundaries and nuclei (cell size 25–35 µm) [Fig. 1(a)]. The stratum spinosum, which is characterized by monomorphic polygonal cells of 15–25 µm in size, appeared to have a honeycomb structure [Fig. 1(b)]. The papillae extending right into the epidermis were represented as dark areas surrounded by basal cells and melanocytes, which in turn appeared bright due to their high content of keratin and melanin [Fig. 1(c)]. Fig. 1Confocal images of horizontal (en face) sections () of the epidermis in healthy skin showing sections of stratum corneum (SC) and stratum granulosum (SG) (a); at the stratum spinosum level showing a typical honeycomb pattern (b); and of the dermal–epidermal junction area showing many papillae surrounded by bright cells (c). 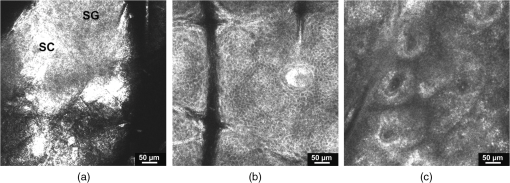 In psoriatic skin, an enlarged stratum corneum was observed as a sign of hyperkeratosis, and the stratum granulosum was either absent or reduced, although the epidermis in total was enlarged with an emphasized stratum spinosum, attributed to acanthosis. The dermal papillae appeared to be elongated and enlarged (Fig. 2). 3.2.Blood FlowReal time in vivo observation in normal skin showed homogeneous blood flow through capillary loops, which were shown to be vastly increased in the blood-filled capillaries of psoriatic skin. 3.3.Capillary Loop StructureIn the healthy skin, each dermal papilla was supplied by a single capillary loop consisting of an ascending and a descending limb. The capillary loop showed a simple hairpin structure. In normal skin, where the horizontal confocal section cuts across dermal papillae, two surfaces of the capillary loop were seen, corresponding to the ascending and descending limb. In the upper part of the dermal papillae, where the ascending limb twists into the descending limb, only one section of the capillary loop was observed (Fig. 3). Fig. 3Capillary loop in healthy skin. (a) Scheme of a healthy dermal papilla supplied by a single capillary loop with simple hairpin structure. (b) Confocal image of a horizontal (en face) section () of a dermal papilla with two surfaces of the capillary loop corresponding to the ascending and descending limb (arrow). 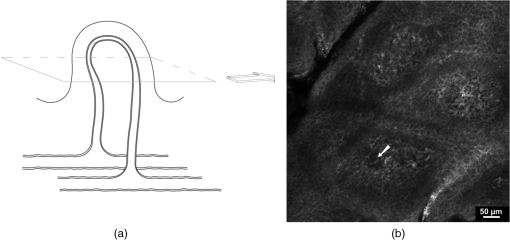 In areas of diseased skin in psoriasis patients, the single loop structure disappeared to a large extent. The vasculature of psoriasis patients appeared as elongated, widened, and tortuous capillary loops within the enlarged dermal papillae. Horizontal CLSM sections obtained at the level of the superficial dermis showed numerous surfaces of the vessels of a capillary loop within a dermal papilla, corresponding to the bushy pattern of the capillary loops (Fig. 4). 3.4.Capillary and Papillary DiameterIn healthy skin, the measured mean outside diameter of the loop capillaries was , and the measured mean papillary diameter was . The capillaries of the papillary loop and dermal papillae were significantly enlarged in psoriatic skin lesions, with a mean outside diameter of the loop capillaries of and a mean papillary diameter of (). 4.DiscussionEarlier observations have shown that alterations of skin microvasculature play an important and most likely active role in the pathogenesis of psoriasis. The loops observed in the capillary vessels of psoriatic plaques have been described as elongated, widened, and tortuous.8 The described microvascular changes in psoriatic plaques occur in the early stages of lesion formation, before any epidermal changes are detectable.22 Focusing the therapeutic goal on these preclinical changes in the dermal vasculature could therefore prevent the development of epidermal changes. Based on the essential role of microvascular changes in the pathogenesis of psoriasis, the aim of this study was to evaluate efficacy of CLSM for monitoring structure and morphology of dermal capillaries and for measuring skin capillary diameters in normal and psoriatic skin. The study was conducted on healthy volunteers and psoriasis patients, differing in age and sex. Age, sex, and body site were shown not to have an influence on the diameter of capillaries in an earlier capillaroscopy study.19 In the present study, we were able to show that the skin of all our examined healthy volunteers demonstrated a simple hairpin structure of the capillary loop. CLSM analysis of the skin of psoriasis patients, on the other hand, consistently showed dilated, elongated, widened, and tortuous capillary loops, as described previously.8 The severity of these structural changes seemed to vary according to severity of the disease. After quantitative analysis, it was possible to verify significant differences concerning the dilatation of the skin capillaries in psoriatic skin compared with normal skin (). Interestingly, the measured average diameter of the capillary loop in normal skin in our study () varied significantly from measurements in earlier studies, which reported average diameter values from 17 µm19 to 30 µm.23 However, the measured value of the loop diameter in this study corresponded well to the average diameter mentioned by Braverman5 (7.5–10 µm), which was based on electron-microscopic findings. This discrepancy may have been a result of differences in choosing measurement points due to difficulty in the identification of the outer borders of capillaries. In our measurement of the outside diameter, we included the thin halo, which represents the endothelial tube of the capillaries. The integrated image analysis software of the confocal laser-scanning microscope does not allow for exact marking of the measurement points. However, the software used in our study allows for precise measurements using the “zoom-in” function. The assumption of Hegyi et al.,19 mentioning that the capillary diameter is largely independent of the depths of the skin, seems to be valid for the majority of capillary loops in the upper portion of the papillae. In deeper layers, however, several other components of skin vasculature become visible, which differ in diameter from the loop capillaries,5 especially in psoriatic skin, due to the disarrangement of the microvasculature. For this reason, we restricted our measurements to the upper layers of the dermal papillae. Considering these potential sources of error, the diameter of the vessels can be seen as a well-quantifiable indicator for the state of psoriatic skin. The diameter of the papillae is also well quantifiable, but because of its less significant role in the pathomechanism of the disease, its evaluation was not the central goal of this study. In clinical practice, visual inspection of the patient’s skin mainly determines the duration of the psoriasis therapy, although it is known that neovascularizations, which persist beyond dermal healing, facilitate the development of recurrences in loco.10 The important role of microvascular changes for maintaining psoriatic skin lesions has been emphasized in earlier capillaroscopic studies.23,24 Continuation of therapy until the capillary structure has regained its normal state could be an advantage and could extend the recurrence-free time. Histological follow-up of “capillary healing” by repeatedly taking biopsies is inappropriate during the kinetic healing process, in the state of inflammation and during the different types of local therapy (baths and ointments). Therefore, compared to conventional biopsies, CLSM may provide a good noninvasive alternative for therapy and follow-up in psoriasis patients, and could provide an excellent tool for the determination of biomorphological endpoints of therapy, delaying the onset of recurrence and minimizing side effects. There are, however, several limitations that must be taken into consideration when investigating psoriasis with CLSM. These may include hyperkeratosis, topical application of keratolytic agents, a reduced or absent granular layer, or significant papillomatosis. Similarly, the papillary dermis may be difficult to visualize due to the decreased amount of light reaching the dermis in lesions with a high degree of parakeratosis, hyperkeratosis, acanthosis, and spongiosis.16 However, further investigations will be required to test the applicability of confocal laser scanning microscopy in the therapeutic management of psoriasis. AcknowledgmentsWe thank the Foundation “Skin Physiology” of the Donor Association for German Science and Humanities for financial support. ReferencesG. K. MenonA. M. Kligman,
“Barrier functions of human skin: a holistic view,”
Skin Pharmacol. Physiol., 22
(4), 178
–189
(2009). http://dx.doi.org/10.1159/000231523 SPPKE6 1660-5527 Google Scholar
E. Proksch,
“The role of emollients in the management of diseases with chronic dry skin,”
Skin Pharmacol. Physiol., 21
(2), 75
–80
(2008). http://dx.doi.org/10.1159/000112957 SPPKE6 1660-5527 Google Scholar
L. Naldi,
“Epidemiology of psoriasis,”
Curr. Drug Targets Inflamm. Allergy, 3
(2), 121
–128
(2004). http://dx.doi.org/10.2174/1568010043343958 1568-010X Google Scholar
H. Valdimarssonet al.,
“Psoriasis: a T-cell-mediated autoimmune disease induced by streptococcal superantigens?,”
Immunol. Today, 16
(3), 145
–149
(1995). http://dx.doi.org/10.1016/0167-5699(95)80132-4 IMTOD8 0167-4919 Google Scholar
I. M. Braverman,
“The cutaneous microcirculation,”
J. Investig. Dermatol. Symp. Proc., 5
(1), 3
–9
(2000). http://dx.doi.org/10.1046/j.1087-0024.2000.00010.x 1087-0024 Google Scholar
I. M. BravermanA. Yen,
“Ultrastructure of the human dermal microcirculation. II. The capillary loops of the dermal papillae,”
J. Invest. Dermatol., 68
(1), 44
–52
(1977). http://dx.doi.org/10.1111/jid.1977.68.issue-1 JIDEAE 0022-202X Google Scholar
A. YenI. M. Braverman,
“Ultrastructure of the human dermal microcirculation: the horizontal plexus of the papillary dermis,”
J. Invest. Dermatol., 66
(3), 131
–142
(1976). http://dx.doi.org/10.1111/jid.1976.66.issue-3 JIDEAE 0022-202X Google Scholar
I. M. BravermanA. Yen,
“Ultrastructure of the capillary loops in the dermal papillae of psoriasis,”
J. Invest. Dermatol., 68
(1), 53
–60
(1977). http://dx.doi.org/10.1111/jid.1977.68.issue-1 JIDEAE 0022-202X Google Scholar
G. Micaliet al.,
“Cutaneous vascular patterns in psoriasis,”
Int. J. Dermatol., 49
(3), 249
–256
(2010). IJDEBB 0011-9059 Google Scholar
M. GordonW. C. JohnsonC. F. Burgoon Jr,
“Histopathology and histochemistry of psoriasis. II. Dynamics of lesions during treatment,”
Arch. Pathol., 84
(5), 443
–450
(1967). 0363-0153 Google Scholar
C. Antoniouet al.,
“Analysis of the melanin distribution in different ethnic groups by in vivo laser scanning microscopy,”
Laser Phys. Lett., 6
(5), 393
–398
(2009). http://dx.doi.org/10.1002/lapl.v6:5 1612-2011 Google Scholar
V. Czaikaet al.,
“Application of laser scan microscopy in vivo for wound healing characterization,”
Laser Phys. Lett., 7
(9), 685
–692
(2010). http://dx.doi.org/10.1002/lapl.201010038 1612-2011 Google Scholar
J. Lademannet al.,
“In vivo laser scanning microscopic investigation of the decontamination of hazardous substances from the human skin,”
Laser Phys. Lett., 7
(12), 884
–888
(2010). http://dx.doi.org/10.1002/lapl.v7.12 1612-2011 Google Scholar
A. PatzeltW. SterryJ. Lademann,
“In vivo measurements of skin barrier: comparison of different methods and advantages of laser scanning microscopy,”
Laser Phys. Lett., 7
(12), 843
–852
(2010). http://dx.doi.org/10.1002/lapl.v7.12 1612-2011 Google Scholar
V. Czaikaet al.,
“Comparison of transepidermal water loss and laser scanning microscopy measurements to assess their value in the characterization of cutaneous barrier defects,”
Skin Pharmacol. Physiol., 25
(1), 39
–46
(2012). http://dx.doi.org/10.1159/000330486 SPPKE6 1660-5527 Google Scholar
M. Ardigoet al.,
“Concordance between in vivo reflectance confocal microscopy and histology in the evaluation of plaque psoriasis,”
J. Eur. Acad. Dermatol. Venereol., 23
(6), 660
–667
(2009). http://dx.doi.org/10.1111/jdv.2009.23.issue-6 JEAVEQ 0926-9959 Google Scholar
E. A. Wolberinket al.,
“Cellular features of psoriatic skin: imaging and quantification using in vivo reflectance confocal microscopy,”
Cytometry B Clin. Cytom., 80
(3), 141
–149
(2011). http://dx.doi.org/10.1002/cyto.b.v80b.3 1552-4949 Google Scholar
S. Kolleret al.,
“In vivo reflectance confocal microscopy of erythematosquamous skin diseases,”
Exp. Dermatol., 18
(6), 536
–540
(2009). http://dx.doi.org/10.1111/exd.2009.18.issue-6 EXDEEY 0906-6705 Google Scholar
J. Hegyiet al.,
“Confocal laser-scanning capillaroscopy: a novel approach to the analysis of skin capillaries in vivo,”
Skin Res. Technol., 15
(4), 476
–481
(2009). http://dx.doi.org/10.1111/srt.2009.15.issue-4 0909-752X Google Scholar
M. Rajadhyakshaet al.,
“In vivo confocal scanning laser microscopy of human skin II: advances in instrumentation and comparison with histology,”
J. Invest. Dermatol., 113
(3), 293
–303
(1999). http://dx.doi.org/10.1046/j.1523-1747.1999.00690.x JIDEAE 0022-202X Google Scholar
L. T. OlsenH. Serup,
“High-frequency ultrasound characterization of normal skin: skin thickness and echographic density of 22 anatomical skin sites,”
Skin Res. Technol., 1
(2), 74
–80
(1995). http://dx.doi.org/10.1111/srt.1995.1.issue-2 0909-752X Google Scholar
M. Goodfieldet al.,
“Investigations of the ‘active’ edge of plaque psoriasis: vascular proliferation precedes changes in epidermal keratin,”
Br. J. Dermatol., 131
(6), 808
–813
(1994). http://dx.doi.org/10.1111/bjd.1994.131.issue-6 BJDEAZ 0007-0963 Google Scholar
S. Hernet al.,
“In vivo quantification of the structural abnormalities in psoriatic microvessels before and after pulsed dye laser treatment,”
Br. J. Dermatol., 152
(3), 505
–511
(2005). http://dx.doi.org/10.1111/bjd.2005.152.issue-3 BJDEAZ 0007-0963 Google Scholar
P. Rosinaet al.,
“Microcirculatory modifications of psoriatic lesions during topical therapy,”
Skin Res. Technol., 15
(2), 135
–138
(2009). http://dx.doi.org/10.1111/srt.2009.15.issue-2 0909-752X Google Scholar
|