|
|
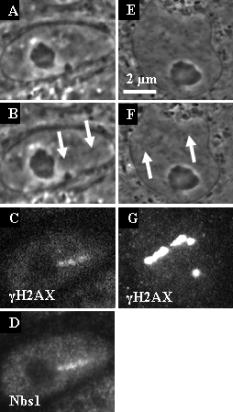
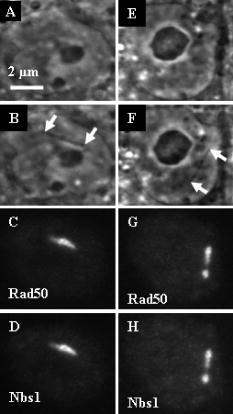
1.IntroductionThe laser microbeam is being used by many laboratories around the world to study DNA damage and repair. These studies generally involve the production of a spatially defined alteration in the cell nucleus, followed by antibody staining for specific DNA repair factors. In these studies, several different lasers and ablation parameters are used: 1. 337-nm UVA wavelength from the laser in combination with a DNA-binding sensitizing agent such as BrdU,1 2. the 337-nm wavelength of the laser used either with BrdU alone or with BrdU and Hoescht at the same time,2 3. the 337-nm laser used alone,1 4. the of the Q-switched nanosecond laser,3 5. the 532-nm picosecond laser,4 and 6. the 364-nm wavelength of the cw argon ion laser.5 In all of these studies, the spatially defined areas of laser irradiation in the nucleus were shown to contain either damage recognition proteins, checkpoint proteins, or DNA repair proteins at varying time points following exposure to the focused laser beam. In this study we utilized a 200-fs near-infrared laser to induce damage in the subnuclear region. We used known markers for DNA double strand breaks (DSBs), including phosphorylation of the histone H2A variant H2AX and recruitment of Nbs1 and Rad50 (components of the trimeric Mre11 complex), to assess the presence of DNA damage and cellular response at the laser-induced damage sites. Phosphorylation of H2AX at serine 139 (termed “ H2AX”) occurs specifically at the DSB damage site and surrounding areas as part of DSB-induced checkpoint signaling.2, 6 The Mre11 complex is one of the earliest DSB factors to be recruited to the damage site and is involved in both DSB checkpoint signaling and repair.7, 8 Our results demonstrated that damage generated by exposure to the ultra-short femtosecond laser pulses at and below the resolution of the optical microscope induces DSB checkpoint response and is recognized by DSB repair factors, indicating that the system can be used to study cellular DNA damage response and repair. 2.Materials and Methods2.1.Cell CultureRat kangaroo, Potorous tridactylus, kidney epithelial cells (PTK1, American Type Culture Collection, [ATCC] CCL 35) and human cystic fibrosis pancreatic adenocarcinoma (CFPAC-1, ATCC CRL 6493) cells were grown in Gibco advanced Dulbeccio’s Modified Eagle Medium (DMEM) F-12 supplemented with L-glutamine, and 3% fetal bovine serum. The cells were incubated at with 5% . Cells were seeded into Rose chambers at a density of cells per mL and allowed to grow for 24 to until semiconfluency, at which time they were used for experimentation at a density of 1 to cells per mL in Rose chambers, as was previously described.9 2.2.Laser IrradiationIrradiation was performed with a Verdi-pumped Mira titanium sapphire laser (Coherent Incorporated, Santa Clara, California) emitting pulses at . Individual laser exposure in the cell nucleus was for at either or using the laser and imaging setup as previously described.10 2.3.Immunofluorescent StainingCells were washed with phosphate buffered saline (PBS) and fixed by 4% paraformaldehyde for 3 to following laser exposure. Cells were permeabilized overnight with 0.1% TritonX in PBS blocking buffer containing 2.5% FBS. After permeabilization, cells were stained for immunofluorescence with antibodies for three DNA repair/recruitment factors: H2AX (Upstate Biotechnology, Temecula, California), a phosphorylated histone that is a DNA-damage marker and two DNA repair factors, Nbs1 (Novus Biologicals, Littleton, Colorado), and Rad50 (GeneTex, San Antonio, California). Zymax Cy3 goat antirabbit and FITC goat antimouse antibodies (Zymed Laboratories, San Francisco, California) were used to detect the primary antibodies, as previously described.11 2.4.ImagingCells were refound by using postirradiation images and grid coordinates. Fluorescence imaging was performed as previously described.10 The width of the line at the site of irradiation was measured by counting pixels of postirradiation images using Image J. 3.Results3.1.Low IrradianceCFPAC-1 and PTK2 cells irradiated at did not appear damaged when viewed with the light microscope [Figs. 1a, 1b, 1e, 1f ]. However, following staining with the H2AX and Nbs1 antibodies, a distinct line of fluorescence was detected that matched the laser exposure region in eight out of eight cells [Figs. 1c, 1d, 1g]. Fluorescence corresponding to the site of irradiation was detected in cells fixed as early as three minutes after irradiation. 3.2.High IrradianceCells irradiated at and stained for Nbs1 and Rad50 (Fig. 2 ) repair factors show strong fluorescence confined to the site of irradiation in 22 out of 23 cells and 12 out of 14 cells, respectively. Of particular interest is the 300-nm-diam narrow phase-dark line of damage [Figs. 2b and 2f]. Both repair factors appear to be localized in the same damage zone. Cells irradiated at this irradiance and antibody stained for H2AX exhibited bright fluorescence throughout the entire nucleus, making it difficult to discriminate the primary damage site as was evidenced at the lower irradiance presented in Fig. 1. 4.DiscussionThe localization of H2AX, Rad50, and Nbs1 to the site of laser irradiation demonstrates that the femtosecond near-IR laser can induce spatially confined DNA/chromatin damage. This damage is probably due to multiphoton and plasma-induced mechanisms of ablation. Resulting damage is nonspecific to the type of molecules, but results in the ablation of molecules within the focal spot of the laser, thus causing multiple DSB surrounding the site of laser exposure. Due to the presence of known DSB markers (gamma H2AX and the Mre11 complex) at the damage sites, laser exposure certainly resulted in DSBs. These results are comparable to those obtained with UV BrdU sensitization1, 2, 12 and with the different laser systems.3, 4 Our observation of early induction of H2AX in the nucleus of irradiated cells is consistent with previous findings that H2AX gets phosphorylated as early as one to three minutes after exposure to ionizing radiation.2 Additionally, our finding that increasing the laser dose from to in the focal spot resulted in spreading of H2AX beyond the region of laser exposure is consistent with previous findings that larger doses of ionizing radiation and UVA laser power resulted in increased H2AX phosphorylation.1, 2 However, the mechanism whereby the damage is conferred to most of the nucleus when only a small region of the nucleus is exposed is not known. Of particular interest is the observation that when the laser dose was reduced to in the focal spot, little or no damage was detected at the light microscope level, but antibody staining was detected at the irradiation site that matched the spatial pattern of laser exposure in the live cell. This demonstrates that the 200-fs laser can produce site-specific damage to the DNA. Immunofluorescence antibody staining for Nbs1 and Rad50 shows specific localization to the site of irradiation similar to that seen in previous studies involving laser microsurgery using both UV and 532-nm lasers. 1, 2, 3, 5, 12, 13, 14 In all cases, the fluorescence is very strong at the site of laser exposure. Studies have shown that Nbs1 localized to the sites of damage as early as after irradiation in human cells.14 Our findings of early localization are consistent with those results. However, of particular interest in our study is the production of a very thin line of damage as seen in the phase-contrast images [Fig. 2b]. This 300-nm-diam line of damage is just at the resolution of the light microscope and compares well with the 100- to 200-nm damage zone in chromosomes that were irradiated with a femtosecond laser and subsequently examined by scanning electron microscopy.15 However, those studies were conducted on dried chromosomes removed from cells, and the studies we report here were conducted on live cells. It is most likely that in those studies, as well as the ones reported here, the damage was caused by multiphoton absorption in the tightly focused spot. Such events were described in laser-irradiated chromosomes more than 25 years ago using a pulsed 10-ns 532-nm laser.16, 17 More recently, multiphoton-induced gene inactivation has been described following irradiation of the ribosomal gene sites on late prophase chromosomes using a 100-ps laser operating at a wavelength of .18 In that study, the chromosomes were sensitized with the nontoxic vital stain ethidium bromide, which had a peak absorption at , very close to the two-photon absorption wavelength of the laser. In summary, we show that DNA/chromatin damage followed by H2AX phosphorylation and double-strand break repair factor recruitment can be induced by a 200-fs near-infrared 800-nm laser. Live cells display spatially defined damage zones as thin as within of laser exposure. These damage regions stain with antibodies for DNA recruitment and repair factors. Irradiation with laser irradiance below the threshold for visible damage in live cells detected by phase-contrast light microscopy reveals antibody staining confined to the exact sites of laser exposure. The general transparency of the cell to the near-IR femtosecond laser, plus the confinement of cell damage only to the focal point where the multiphoton and other nonlinear physical events may occur, make this laser ideal for future studies on DNA damage and repair mechanisms. Note added in proof: Recently, a similar femtosecond laser was used to study non-homologous end-joining factors [ Marc, Proc. Nat. Acad. Sci. USA 103, 18597–18602 ]. AcknowledgmentsThis work was supported by grants from the United States Air Force (AFOSR F9620-00-1-0371), the National Institute of Health (NIH RR 14892), and the Department of Defense Breast Cancer Research Program (DAMD17-03-1-0436 to Yokomori). A special thanks to the Arnold and Mabel Beckman Foundation and to Minorities Access to Research Careers (NIH GM 69337). ReferencesC. Lukas,
J. Falck,
J. Bartkova,
J. Bartek, and
J. Lukas,
“Distinct spatiotemporal dynamics of mammalian checkpoint regulators induced by DNA damage,”
Nat. Cell Biol., 5
(3), 255
–260
(2003). 1465-7392 Google Scholar
E. P. Rogakou,
C. Boon,
C. Redon, and
W. M. Bonner,
“Megabase chromatin domains involved in DNA double-strand breaks in vivo,”
J. Cell Biol., 146
(5), 905
–916
(1999). 0021-9525 Google Scholar
J. S. Kim,
T. B. Krasieva,
V. LaMorte,
A. M. Taylor, and
K. Yokomori,
“Specific recruitment of human cohesin to laser-induced DNA damage,”
J. Biol. Chem., 277
(47), 45149
–45153
(2002). 0021-9258 Google Scholar
B. P. Chen,
D. W. Chan,
J. Kobayashi,
S. Burma,
A. Asaithamby,
K. Morotomi-Yano,
E. Botvinick,
J. Qin, and
D. J. Chen,
“Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks,”
J. Biol. Chem., 280
(15), 14709
–14715
(2005). 0021-9258 Google Scholar
K. Negishi,
S. Higashi,
T. Nakamura,
C. Otsuka,
M. Watanabe, and
T. Negishi,
“Oxidative DNA damage induced by 364-nm UVA laser in yeast cell,”
Genes Environ., 28
(2), 74
–76
(2006). Google Scholar
E. P. Rogakou,
D. R. Pilch,
A. H. Orr,
V. S. Ivanova, and
W. M. Bonner,
“DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139,”
J. Biol. Chem., 273
(10), 5858
–5868
(1998). 0021-9258 Google Scholar
M. F. Lavin,
“The Mre11 complex and ATM: a two-way functional interaction in recognising and signaling DNA double strand breaks,”
DNA Repair, 3
(11), 1515
–1520
(2004). 1568-7864 Google Scholar
B. E. Nelms,
R. S. Maser,
J. F. MacKay,
M. G. Lagally, and
J. H. Petrini,
“In situ visualization of DNA double-strand break repair in human fibroblasts,”
Science, 280
(5363), 590
–592
(1998). 0036-8075 Google Scholar
M. W. Berns,
E. Botvinick,
L. Liaw,
C. Sun, and
J. Shah, Cell Biology a Laboratory Handbook, 352
–353 Copenhagen(2006). Google Scholar
N. M. Wakida,
C. S. Lee,
E. L. Botvinick,
L. Z. Shi,
A. Dvornikov, and
M. W. Berns,
“Laser nanosurgery of single microtubules reveals location dependent depolymerization rates,”
J. Biomed. Opt., 12
(2), 024027
(2007). 1083-3668 Google Scholar
T. T. Paull,
E. P. Rogakou,
V. Yamazaki,
C. U. Kirchgessner,
M. Gellert, and
W. M. Bonner,
“A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage,”
Curr. Biol., 10
(15), 886
–895
(2000). 0960-9822 Google Scholar
S. Bekker-Jensen,
C. Lukas,
R. Kitagawa,
F. Melander,
M. B. Kastan,
J. Bartek, and
J. Lukas,
“Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks,”
J. Cell Biol., 173
(2), 195
–206
(2006). 0021-9525 Google Scholar
L. Lan,
S. Nakajima,
Y. Oohata,
M. Takao,
S. Okano,
M. Masutani,
S. H. Wilson, and
A. Yasui,
“In situ analysis of repair processes for oxidative DNA damage in mammalian cells,”
Proc. Natl. Acad. Sci. U.S.A., 101
(38), 13738
–13743
(2004). 0027-8424 Google Scholar
C. Lukas,
F. Melander,
M. Stucki,
J. Falck,
S. Bekker-Jensen,
M. Goldberg,
Y. Lerenthal,
S. P. Jackson,
J. Bartek, and
J. Lukas,
“Mdc1 couples DNA double-strand break recognition by Nbs1 with its H2AX-dependent chromatin retention,”
EMBO J., 23
(13), 2674
–2683
(2004). 0261-4189 Google Scholar
K. Konig,
I. Riemann, and
W. Fritzsche,
“Nanodissection of human chromosomes with near-infrared femtosecond laser pulses,”
Opt. Lett., 26
(11), 819
–821
(2001). 0146-9592 Google Scholar
M. W. Berns,
J. Aist,
J. Edwards,
K. Strahs,
J. Girton,
P. McNeill,
J. B. Rattner,
M. Kitzes,
M. Hammer-Wilson,
L. H. Liaw,
A. Siemens,
M. Koonce,
S. Peterson,
S. Brenner,
J. Burt,
R. Walter,
P. J. Bryant,
D. van Dyk,
J. Coulombe,
T. Cahill, and
G. S. Berns,
“Laser microsurgery in cell and developmental biology,”
Science, 213
(4507), 505
–513
(1981). 0036-8075 Google Scholar
P. P. Calmettes and
M. W. Berns,
“Laser-induced multiphoton processes in living cells,”
Proc. Natl. Acad. Sci. U.S.A., 80
(23), 7197
–7199
(1983). 0027-8424 Google Scholar
M. W. Berns,
Z. Wang,
A. Dunn,
V. Wallace, and
V. Venugopalan,
“Gene inactivation by multiphoton-targeted photochemistry,”
Proc. Natl. Acad. Sci. U.S.A., 97
(17), 9504
–9507
(2000). https://doi.org/10.1073/pnas.97.17.9504 0027-8424 Google Scholar
|